
Introduction
The world of fragrances comprises of an immense variety of distinct compounds and infinite possibilities for compositions both man-made and natural. The ingredients of fragrance compositions may be complex mixtures of more or less volatile substances that were extracted from natural sources such as blossoms or leaves.
However, fragrance ingredients may also be highly pure single components, either isolated from nature and then enriched and purified by sophisticated techniques, or laboratory made. The latter process may involve purely chemical synthesis methods as well as biotechnological methods, which utilize microorganisms for the production of desired volatiles.
Natural fragrance ingredients
Of course, consumers love the idea that rose gardens and lavender fields are the cradle of their perfumes, which is often the case for high quality, high priced products. While rose and lavender are relatively straightforward to be grown, harvested and processed to high quality natural fragrance ingredients, this is not the case for all desired volatiles. A famous example is the smell of sandalwood: sandalwood oil is so much sought after that sandalwood trees were heavily harvested and became a rarity on our planet. They now belong to the vulnerable category of the IUCN Red List. 1 In this sense, harvesting natural resources for the production of our favorite fragrances is not always sustainable and sometimes even impossible.
The potential of biotechnology
Microorganisms such as bacteria, yeasts, fungi and algae are fast growing, demand little space and they are able to produce a huge variety of useful compounds. These organisms grow on natural sources and when they finished their productive life, the remnants are compostable biomass. Production can be done at any time, independently of all the conditions that threaten traditional harvests, like whether, temperature, suboptimal water supply, insects, pathogens, pollution, etc. Such biotech-processes can basically be set up at any place with enough water and energy, are independent of seasons, and they typically deliver predictable, and reproducible amounts and qualities of the desired product.
From the ecological viewpoint, bioprocesses help to avoid excessive land-use for non-food crops, monocultures and the associated need for herbicides, pesticides and fertilizers as well as their negative impact on biodiversity.

Sandalwood Oil
In case of Sandalwood (Santalum album L.), the challenge was to provide a sustainable replacement for Sandalwood oil, which is highly valued for its unique warm, woody, balsamic and milky tonality. The oil has also a history of being used in dermo-cosmetic applications because it shows antimicrobial, antioxidant and soothing effects. The chemical structures of the two major constituents of the natural oil are (+)-(Z)-a-santalol and (-)-(Z)-b-santalol, representing 55 – 60% and 25–30%, with the latter compound being the characteristic principle. The first step in the development of an efficient biocatalyst was to understand how the desired compounds are biosynthesized in a Sandalwood tree. This information is ultimately encoded on genetic level and was deciphered as two key enzymes, namely a terpene synthase which cyclizes farnesyl-diphosphate to santalene, and a P450-monooxygenase with the necessary reductase which together decorate santalene with an alcohol functionality (Scheme 1). 2 By introducing these genes into the bacterium Escherichia coli, the desired santalols could be produced in a bioreactor for the first time. 3 Since the amount of available farnesyl-diphosphate in E. coli was low, the production of this essential precursor molecule was boosted by introduction of heterologous mevalonate pathway genes. 4 The product resembles the Sandalwood oil isolated from Sandalwood trees very well and is marketed under the name of Dreamwood® today. 2
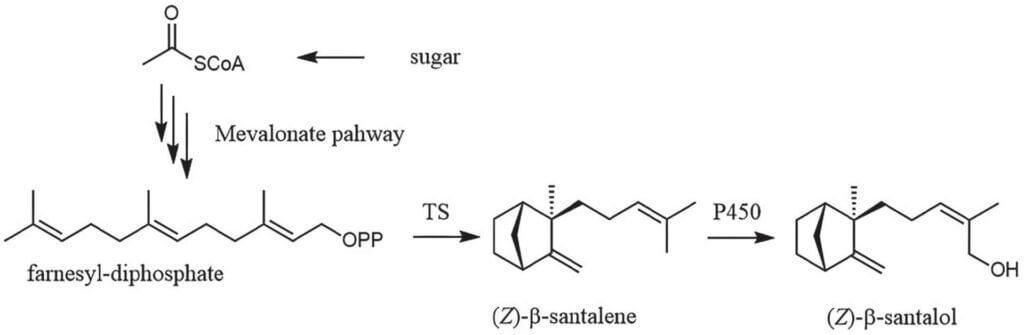
Scheme 1. De novo biosynthesis of the key terpenoid of Sandalwood Oil. E. coli is used as an engineered microbial host that produces (Z)-bsantalol and three other terpenoids from simple sugar in a bioreactor.
Therefore, mevalonate pathway genes were introduced, in addition to Santalum album L. terpene synthase (TS), and a hydroxylase system (P450). For simplicity, only the most characteristic compound is depicted.
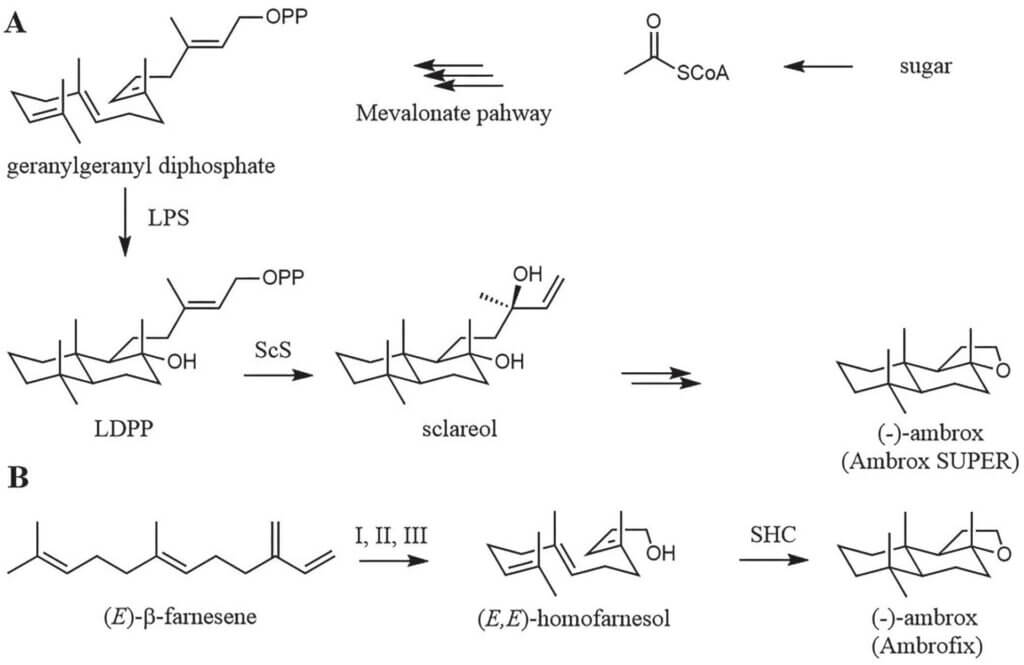
Scheme 2. Production strategies for (-)-ambrox; A) Route to Ambrox® Super that combines synthetic biology with chemistry: De novo biosynthesis of sclareol with the key enzymes (13E)-8a-hydroxylabden-15-yl diphosphate (LDPP) synthase (LPS) and sclareol synthase (ScS) is followed by chemical steps; B) chemoenzymatic route to Ambrofix® from renewable fanesene. Introduction of
the hydroxy functionality by amination and chlorination (I), alkoxycarbonylation (II) followed by hydrolysis and reduction (III) and finally enzymatic cyclization of (E,E)-homofarnesol by a squalene hopene cyclase (SHC).
Ambergris
Ambergris is another valuable and expensive natural product with a sweet, earthal note. Ambergris is a remnant of sperm whale stomachs that originally has a marine, fecal odor, but as it is “aging” in seawater, it develops the desired olfactory characteristics.
The material is finally deposited on sunny beaches where precious stone-like pieces may be found by those who search for them. It is quite obvious that an alternative way to obtain the
major constituents of Ambergris is on high demand. Ambrox is the key molecule with characteristic and memorable ambery and woody odour reminiscent of clary sage and tobacco.5

The traditional route to synthesize ambrox starts from sclareol, which is isolated from the flowers and leaves from clary sage (Salvia sclarea), 4 followed by a multi-step synthesis. 6 To avoid the need to grow, harvest and process clary sage, a highly efficient E. coli strain was developed for the production of sclareol from simple sugars in the bioreactor (Scheme 2A). 4 Alternatively, a reaction sequence was established that starts with (E)-b-farnesene, a compound that is produced by fermentation on large scale because its properties allow its utilization as a renewable jet fuel. Farnesene is converted to its corresponding alcohol in a five-step reaction sequence and finally an engineered squalene hopene cyclase catalyzes the domino-reaction that converts the simple linear alcohol into the chiral, tricyclic molecule (-)-ambrox in a single step (Scheme 2B).6
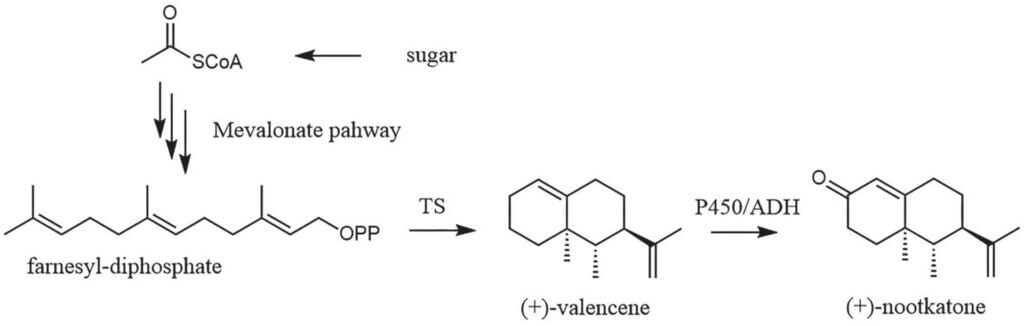
Scheme 3. De novo biosynthesis of valencene and nootkatone by cutting-edge fermentation technology. The key enzymes is terpene synthase (TS) to form valencene. When nootkatone is targeted, additionally, a hydroxylase system (P450) and an oxidoreductase (ADH) mediated reaction are required.
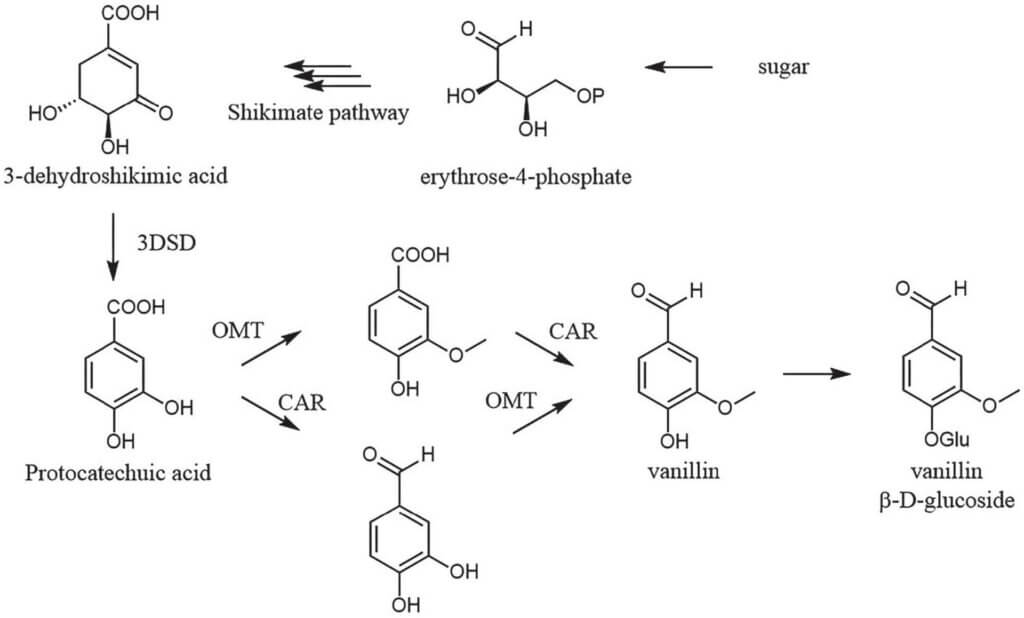
Scheme 4. De novo biosynthesis of vanillin. The key enzymes are 3-dehydroshikimate dehydratase (3DSD), carboxylic acid reductase (CAR) and an O-methyl transferase (OMT).
Valencene and Nootkatone
Two further commercial odorants that are being produced by biotechnology are valencene (Isobionics® Natural Valencene) and nootkatone (Isobionics® Nootkatone). In fact, nootkatone is an oxidized form of (+)-valencene. The latter compound is present in various citrus species and has a woody citrus characteristic, while nootkatone is particularly associated to grapefruit.7 The biosynthetic pathways resemble in the first part very much those of the above explained examples (Scheme 3). The branching point is the particular terpene synthase that nature has designed to produce signature terpenes and labs have further evolved to meet the requirements of industrial scale production processes. Not only the key enzymes but also the production host need to be chosen wisely or improved by metabolic engineering to direct the carbon flux towards the desired products. In the context of valencene and nootkatone production, different yeast strains8, 9 were evaluated in addition to E. coli and the proteobacterium Rhodobacter sphaeroides.7

Vanilla
The scent of vanilla has a mood-lifting and relaxing effect. Vanillin is therefore a popular ingredient in all kinds of products, including cosmetics. For reasons of very limited supply of the natural vanilla pods, biotechnologists around the world are developing concepts on how to access “biovanillin” in a sustainable way, possibly by repurposing waste streams of food production. A breakthrough in this arena was the development of yeast strains for the production of vanillin glucoside – a stable derivative of vanillin.10 Specifically, the intermediate 3-dehydroshikimic acid from the shikimate pathway is redirected to form protocatechuic acid. A methyl group is introduced by an O-methyl transferase and the acid functionality is reduced to aldehyde (Scheme 4). The enzyme class responsible for the latter step in this reaction sequence has been in the spotlight not only for the preparation of vanillin,11,12 but also for a number of other aldehydes with olfactory properties and significance as flavor and fragrance ingredients.13,14 In our labs, for example, we focused on the preparation of heliotropin15 and fatty aldehydes.16
The examples outlined above are only the tip of the iceberg regarding possibilities of how to implement biotechnology to create sustainable molecules. Consumers are thriving for pleasant sensations, and hence the market demand for organoleptic compounds – both known and fancy new – is increasing. The traditional answer to this demand is chemistry, which can be fast and efficient.17,18 In recent years, chemical production is fortunately transitioning towards sustainable production and this involves more and more the implementation of biocatalytic steps5 or fully biocatalyzed processes3,7 which qualify the resultant products to be marketed as natural ingredients.

Acknowledgements
The COMET center: acib: Next Generation Bioproduction is funded by BMK, BMDW, SFG, Standortagentur Tirol, Government of Lower Austria and Vienna Business Agency in the framework of COMET – Competence Centers for Excellent Technologies. The COMET-Funding Program is managed by the Austrian Research Promotion Agency FFG.
References
- A. N. Arun Kumar, G. Joshi, H. Y. Mohan Ram, Curr. Sci. 2012, 103, 1408–1416.
- M. Schalk, A. Taglieber, L. Daviet, Perfum. Flavorist 2020, 45:5.
- L. Daviet, Y. Huang, V. Harraca, M. Schalk, M. Bandera, Biochemically Produced Sandalwood Oil, 2021, WO 2021/130241 A1.
- M. Schalk, L. Pastore, M. A. Mirata, S. Khim, M. Schouwey, F. Deguerry, V. Pineda, L. Rocci, L. Daviet, J. Am. Chem. Soc. 2012, 134, 18900–18903.
- E. Eichhorn, C. Baumgartner, M. Biermann, Chimia (Aarau). 2023, 77, 384–389.
- E. Eichhorn, F. Schroeder, J. Agric. Food Chem. 2023, 71, 5042–5052.
- J. Beekwilder, A. van Houwelingen, K. Cankar, A. D. J. van Dijk, R. M. de Jong, G. Stoopen, H. Bouwmeester, J. Achkar, T. Sonke, D. Bosch, Plant Biotechnol. J. 2014, 12, 174–182.
- T. Wriessnegger, S. Moser, A. Emmerstorfer-Augustin, E. Leitner, M. Müller, I. Kaluzna, M. Schürmann, D. Mink, H. Pichler, Fungal Genet. Biol. 2016, 89, 114–125.
- X. Li, Q. An, S. sha Qu, J. N. Ren, G. Fan, L. L. Zhang, S. Y. Pan, Int. J. Biol. Macromol. 2022, 220, 1031–1048.
- E. H. Hansen, B. L. Møller, G. R. Kock, C. M. Bünner, C. Kristensen, O. R. Jensen, F. T. Okkels, C. E. Olsen, M. S. Motawia, J. Hansen, Appl. Environ. Microbiol. 2009, 75, 2765–74.
- M. Horvat, G. Fiume, S. Fritsche, M. Winkler, J. Biotechnol. 2019, 304, 44–51.
- J. Park, H. S. Lee, J. Oh, J. C. Joo, Y. J. Yeon, Biochem. Eng. J. 2020, 161, 107683.
- M. Winkler, J. G. Ling, ChemCatChem 2022, 14, e202200441.
- L. Schober, H. Dobiašová, V. Jurkaš, F. Parmeggiani, F. Rudroff, M. Winkler, Flavour Fragr. J. 2023, 38, 221–242.
- D. Schwendenwein, G. Fiume, H. Weber, F. Rudroff, M. Winkler, Adv. Synth. Catal. 2016, 358, 3414–3421.
- M. Horvat, M. Winkler, ChemCatChem 2020, 12, 5076–5090.
- G. Sagorin, E. Cazeils, J. F. Basset, M. Reiter, Chimia (Aarau). 2021, 75, 780–787.
- D. Dylong, P. J. C. Hausoul, R. Palkovits, M. Eisenacher, Flavour Fragr. J. 2022, 37, 195–209.
Picture source: #Karl-Heinz Simon



