Abstract
Silicone chemicals exhibit unique surface as well as bulk properties that make them highly desirable for cosmetic applications. These include chemical inertness, biocompatibility, lubricity, comfort feel, slippery feel, compatibility with various formulations, hydrophobicity,
hydrophilicity, broad synthetic molecular design options, and unique ability to combine several properties into one single molecule.
These properties are possible due to unique molecular characteristics such as low surface energy, flexible bonds, high bond energy; high thermally stability. In recent times, these characteristics are becoming more relevant when viewed from the standpoint of environmental
compatibility and overall sustainability. As an example, at equal grammage, silicones exhibit a 70% lower carbon footprint than their organic counterpart.
Most importantly, silicone molecules represent dual orgno-inorgano properties due to the presence of inorganic bond Si-O-Si and organic bond Si-C, offering mixed organic and inorganic properties. As a result, the chemist can design molecules to combine several unique properties into a single molecule and not possible through traditional organic chemistry synthesis. These molecules offer far better value when you view them from the point of view of sustainability, recycling and environmental compatibility.
Thus, it is possible to create a silicone molecule that would offer super hydrophobic surfaces or super-spreadable hydrophilic surfaces simply by modifying the synthetic route. Additionally, hydrolysation technology allows us to chemically combine organic moieties with Siloxane backbone (“Si–C” bond), offering unique hybrid properties and superior environmental compatibility and sustainability options.
Thus, there are immense possibilities to create hybrid molecules that would offer superior properties targeted for specific applications.
The present state of technology has barely scratched the surface concerning the type of molecules that have offered great promise to the cosmetics industry.
The present paper will discuss silicone molecular engineering along with its applicability in cosmetics formulation and its implication for sustainability and environmental compatibility.
Introduction
Silicones have versatile properties that makes them uniquely suitable for personal care applications 1. Specifically, their biocompatibility, inertness, lubricity, low surface energy combined with favourable sustainability parameters make them a preferred choice for personal care applications. Silicone also offers a broad range of synthesis options where multiple properties could be combined at the molecular level by making it possible to create molecules for specific applications. While personal care industry is well aware of the unique properties of Silicones, its penetration into the value-added cosmetic formulations have been modest and thus offers a vast opportunity to expand its application to a broad range of cosmetic formulation in the near future. The reason for the slower penetration has been due to compatibility issues with organic compound formulations. However, such compatibility limitation can easily be resolved by focusing on synthesis options to combine organic moieties with Silicone compounds at the molecular level. Following are the key attributes of silicones for personal care applications;
- Excellent spreading and penetrability due to lower surface and interfacial tensions of Silicones.
- Excellent Bio-compatibility and overall inertness and stability with respect to pH; temperature due to unique oragano – inorganic character.
- Ease of processing due to broad liquid state range (-55 °C to 320 °C) and molecular weight from 300 to 300,000.
- Higher level of oxygen breathability of silicone films due to 5x higher oxygen solubility hydrodynamic.
- Superior hydrodynamic lubricity, soft touch and non-sticky characteristics due to lower cohesive forces and lower surface energy.
- Broad synthesis options to combine properties at the molecular level while lowering compatibility issues.
- Excellent moisture management in bulk or on the surface due to its lower surface energy and hydrophobic character.
- Excellent shine and gloss on surfaces due to higher refractive index and optical clarity.
There are many other attributes to silicones that can be further expanded. However, the above stated are key attributes that have been well understood and are been used in diversified applications.
Based on these attributes there are four application segments where Silicone role has been demonstrated and well understood 2, 3.
Needless to say, a wide range of personal care products contains silicones. It has been used as a functional ingredient in personal care. Sunscreen including lotions, skin creams, and foundations, silicone increases water repellency, raises the sun protection factor (SPF), and lessens the stickiness of organic sunscreens. In hair care products, such as shine sprays, conditioners, frizz serums and shampoo, silicone offers superior hydration, adds shine, controls frizz, make it easier to detangle hair, and prevents hair from further damage. In skin care products like anti-aging serums, lotions, and creams, acne treatments, give the skin a silky smooth texture and conceal fine wrinkles to give off the qualities of a healthier and younger appearance. In deodorant gels and anti-perspirant, roll-ons and sticks, aerosols, increase skin sensation and enable easy glide while lowering the antiperspirant salt’s whiteness and tack. Color cosmetics like lip colors and lipsticks, eye shadows, foundations, primers and powders, they deliver long-lasting performance and color intensity, homogeneity, nontransfer, luminance, and comfort.
Both surface and bulk phenomena together make up this unique property profile of silicones. Such as low surface tension, spreading and “creep” behavior, moderate water interfacial tension,
variety of structural configurations, low surface viscosity, large free volume, the low activation energy of viscous flow, low glass transition temperature, the small temperature variation of
physical constants, liquid nature at the high molecular weight (linear polymers), low freezing and pour points, low boiling points (oligomers), high compressibility, high permeability to gas and low molecular weight species, excellent weather resistance and last but not the least is a low environmental hazard. In the current scenario, these qualities have gained increased significance
when considered in terms of overall sustainability and compatibility with the environment 4. For instance, silicones have a much lesser carbon footprint than their organic counterpart at the
same grammage 5.
Therefore, this article presents silicones in light of their molecular engineering, its applicability in cosmetics formulation and their implication for sustainability and environmental compatibility.
Also, describe the silicone degradation processes in the environment and any possible ecological effect on the land, marine, and environmental segments.
Silicone chemistry and its special features
There are three silicone synthesis techniques: the Grignard approach, the Rochow method using a silicon reaction with an alkyl chloride, and the addition method 6. Normal silicones and organic
polymers are incompatible, but if a silicone has a bigger organic group attached, the silicone’s characteristics are changed and it can be combined with organic polymers. This technology offers a novel way to combine the special qualities of silicone with conventional organic compounds. The hydrosilation reaction process is used to incorporate organic groups into silicone’s backbone 1, 3, 7, 8. Without a doubt, the most fundamental reaction that has broadened the range of uses for silicones is hydrosilation. In the chemical process of hydrosilation, a platinum catalyst is used to add an allyl group to the SiH group on the siloxane backbone.
Carbon chemistry and silicone chemistry can be combined to produce polymers with distinct features and superior performance characteristics 8, 9.
By reducing potassium fluorosilicate with potassium, Berzelius finds silicone in 1824:
4K + K2SiF6 → Si + 6KF (1)
Further, reacting silicone with chlorine gives a volatile compound later identified as tetrachlorosilane:
SiCl4 : Si + 2Cl4 → SiCl4 (2)
Tetraethylsilane, the first silicone organic chemical, is created by Friedel and Craft in 1863:
2 Zn (C2H5)2 + SiCl4 → Si(C2H5)4 + 2 ZnCl2 (3)
Ladenburg made the observation in 1871 that diethyldiethoxysilane produces an oil that only decomposes at extremely high temperatures when it is present with a diluted acid.
Between 1901 and 1930, Kipping used Grignard processes to synthesise different silanes and hydrolyze chlorosilanes to produce big molecules, laying the groundwork for organosilicone
chemistry. Dow Corning’s demonstration of the silicone resins’ high thermal stability and electrical resistance, along with General Electric’s discovery of a direct method (the Racow process) to produce silicones directly from silicon and methyl chloride while using copper catalyst, led to the commercialization of silicones in the 1940s. As of now, over 7000 specialty products are based on a silicone. It is because of the exceptional physicochemical and
mechanical properties of silicone-based materials 10.
Physicochemical properties
Due to several physical characteristics, silicone polymers have the most flexible backbone structures. This is accurate for a number of crucial reasons. The organic portion in polydimethylsiloxane (PDMD) is the methyl group. The intermolecular forces between
molecules are what determine a substance’s surface energy, and in the case of the methyl group, these forces are almost as weak as they can be among hydrocarbon molecules, only aliphatic
fluorocarbon groups are weaker. The structure of a PDMS is given in Fig. 1.
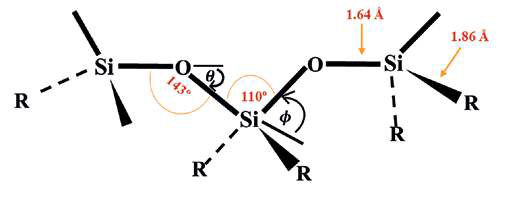
Fig. 1. Schematic representation of siloxane backbone
The increased size of silicon atoms relative to the small oxygen atoms is one of the fundamental structural characteristics influencing the behaviour of siloxane chains. Additionally, the siliconoxygen (Si-O) bond length (1.64 Å) is considerably longer than the carbon-carbon (C-C) bond length (1.53 Å) 1, 11, 12. As a result, steric interferences are greatly reduced. Table 1 shows the physicochemical properties of PDMS structure. Additionally, the Si-O bond can be seen as having a 50% ionic character due to the stark contrast between Si and O electronegativities. As a result, the Si-O bond’s dissociation energy (108 kcal mol-1) is substantially higher than the C-C bond’s dissociation energy (83kcal mol-1). Therefore, the Si-O polymer backbone is sensitive to electrophilic and nucleophilic assault while being thermally stable. Additionally, the all-trans version of the molecule forms a cyclical projection that circles back on itself after around 11 repetition units and transforms into a helical structure for long chains due to the inequality
of the Si-O-Si bond angle (143°) to O-Si-O bond angle (110°). Consequently, because of these structural characteristics, the dynamic flexibility of the siloxane greatly increases8, 11, 13.
Special characteristics of silicone
Compared to carbon compounds, silicon sp3 hybridized bonds are not directionally rigid. Because silicone with electronegative atoms similar to nitrogen and oxygen form bonds with a significant ionic component 15. Due to it, the inorganic siloxane backbone (-Si–O–Si-) is the most flexible polymer backbone, and this enables the best possible presentation and arrangement of the methyl groups. The development of the PDMS molecule’s helical
configuration, in which the oxygen faces inward and the methyl groups face outward, further strengthens these effects. This unusual structure has inert methyl groups that are exposed to the environment, like nano-springs. Because of this, PDMS offers one of the lowest energy surfaces available, only being surpassed by more expensive fluorocarbon polymers 14. In comparison to water or many organic liquids, they also show much higher solubility for oxygen. Many silicone applications, including adhesives, emulsions, antifoams, and surfactants, are due to the unusual surface behaviour and molecular flexibility that the Si-O-bond imparts 15–17. The silicone product’s ability to adhere to another material is crucial in many applications (Fig. 2). By carefully designing and creating silicone products that connect directly with the substrate (skin or hair), whether the silicone is employed as a coating, sealant, or adhesive, a low surface energy polymer is adhered to another material 17–19.
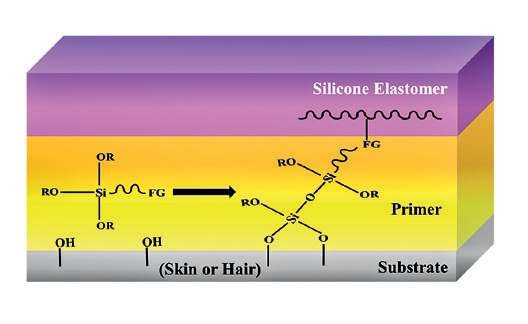
Fig. 2. Adhesion between silicone elastomers and substrates
Silicone polymers are frequently employed in applications and processes that involve water. Most silicone polymers cannot dissolve in water. They are often created as an emulsion for aqueous delivery, which is a dispersion of tiny silicone oil droplets in an aqueous surfactant solution 20. This type of silicone polymer formulation is frequently utilised in cosmetic and personal care products. Normally, silicone adds functionality to hair conditioners and serums. Although inorganic silicon is a crucial component for bone growth, silicones are only found in man-made materials; no plausible explanation for the origin of naturally occurring organosilicone
compounds has ever been found. The peculiar qualities of silicones also explain why these materials are used in modern technology; these peculiar qualities. Many silicones exhibit better thermal characteristics than traditional organic polymers.
Some silicone elastomers maintain their flexibility at -100 °C and long-term property retention at 200 °C 17. The majority of popular silicone polymers are quite permeable to water vapour and other gases but are not wetted by liquid water. Their resistances to ultraviolet and other radiation means that they are compatible in adverse climatic conditions and hence silicone based sun cream and cosmetic products have significant demand in personal care sector 21.
Silicones in cosmetic industries and its applications
Silicones have a high level of biocompatibility and added sensory appeal, silicones are a popular choice for many personal care products 22, 23. Personal care applications include a variety of silicones, including silicone elastomer dispersions and resins, as well as cyclic, linear, or organo-functional polydimethylsiloxanes (PDMS). This broad variety of compounds confers qualities including good spreading, film formation, wash-off resistance, skin feel, volatility, and permeability, which have an impact on the performance of practically every type of cosmetic product. Silicones are crucial elements because of their distinctive chemical and physical characteristics. These adaptable materials, which are most known for their aesthetic qualities, enhance the performance of numerous cosmetics, sunscreens, and skin care products.
They aid in the delivery of pigments and other skin-care compounds, boost UV protection, and increase the stability of anti-aging substances.
In the 1950s, silicones were utilised in skin care products. Initially dimethicone, the basic silicone fluid were used in personal care application. These linear polymers are liquid over a wide range of molecular weights 24. For its emollient qualities and capacity to enhance the skin-feel of many different skin care formulations, dimethicones continue to be significant. Another significant class of silicones entered the market in the late 1970s. Lowviscosity, volatile silicone fluids called cyclomethicones serve as cosmetic solvents 25. They are especially well suited for use in conjunction with other silicones and as carriers for various active substances.
Since the 1980s, silicone manufacturers have been responding to the rising demand for silicones in skin care products by creating a wide range of novel materials, which has expanded their use. Numerous brand-new silicones were derived from dimethicones, wherein the silicone polymer’s backbone was given particular functional groups. For instance, the production of nonionic silicone surfactants, which are helpful as emulsifiers, foam stabilisers, and wetting agents, can be achieved by grafting hydrophilic polyethylene oxide chains to the dimethicone backbone.
Phenyl groups were added to the silicone backbone to make fluids with a higher refractive index and improved compatibility with cosmetic waxes, resulting in the creation of another class of
silicones. These phenyl silicones are excellent for creating a high gloss covering in colour cosmetics like lipsticks4, 26, 27.
Silicone elastomers, which are created by cross-linking dimethicone polymers to create elastomeric solids with characteristics very different from dimethicone fluids, are one of the newest and fastest-growing classes of silicones utilised in skin care applications 27, 28. The silicone network stiffens as the cross-linking intensity rises. Silicone resins are the most extreme forms of crosslinked silicone polymers, despite the fact that they are created using a different method. The rigid materials produced by silicone resins, which have a close, three-dimensional structure, can be used to create tough, long-lasting films 6.
In particular, leave-on skincare products and other types of hair care products use a variety of silicones in their formulations. Silicones are referred to by numerous names and are altered into a range of different formulae in order to perform the specific activities that are required of them, such as waterproofing, holding moisture, adhering colour pigments, protecting our hair, and imparting smoothness. Additionally, they add smoothness and make skincare product applications feel silky, so the skin is not pulled when they are applied and there is no greasy or sticky sensation. Our deodorants get a velvety feel from them, enabling them to dry quickly, and water-resistant sunscreens can remain on our skin even when we perspire or get wet 29.
Furthermore, silicones offers excellent breathability to the skin. Because, it is impossible for silicones to suffocate skin due to their molecular structure. Large molecules with vast spaces between them give silicones their special molecular structure, which also explains why they don’t typically feel heavy or occlusive despite providing protection against moisture loss and enabling them to form a permeable barrier 8, 30. It’s interesting to note that silicone has been demonstrated to be effective in reducing dryness and flaking caused by typical anti-acne active ingredients like benzoyl peroxide and topical antibiotics. Additionally, silicones are occasionally utilised as fillers to minimise the look of acne scars, which is unthinkable if silicone were a pore-clogging component. The fact that most silicones are volatile from a chemistry perspective is perhaps the most revealing factor in why they do not clog pores and cause acne (or blackheads)31.
It means silicones are the backbones for cosmetic products, especially for conditioning our hair. The most prevalent ones are dimethicone and cyclomethicone, which are found in most shampoos, conditioners, treatments, and styling products 23. They can fix damage indications, fill up craters and crevices on the surfaces of hair, restore water resistance to harmed places, and stop fresh damage from happening (Fig. 3).
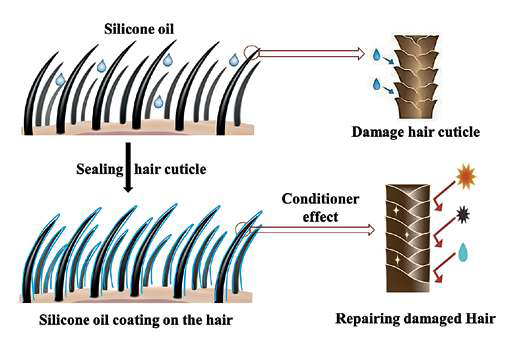
Fig. 3. Schematic representation of the process of silicone emulsion absorption to the hydrolipid layer of hair
The majority of cosmetics, including foundation, eyeshadow, blush, and liquid and pencil eyeliners contain at least one type of silicone 6. Liquid foundation’s silicones make it flexible on the face and preserve its fresh, dewy appearance. Without them, you can end up with dry powder on your face, which is more prone to develop into laugh or wrinkle lines.
Silicones are added to a composition to make it more resistant to water. This is especially helpful for foundations, powders, and sun protection that advertise that they are water-resistant and waterproof.
Elastomer powders have various visual qualities that range from silky smooth to powdery with a dry soft feel 28. They were especially important in a new form of treatment known as “skin primer”. To achieve perfect skin, silicone-based primers were essential. Silicones have been utilised in skin care products for more than 50 years and are still widely used today 32. Hence,
the global silicone cosmetics market was over USD 4 billion in 2019 and is expected to grow with a USD 5.2 billion in 2024 over the forecast period (Fig. 4). Prevalent use of silicone (PDMS), owing to its increasing investment in cosmetics and personal care along with the medical industry are contributing factors driving the global silicone polymer market 33.
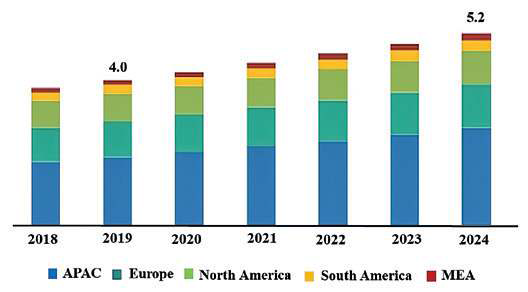
Fig. 4. Silicone (PDMS) market size in cosmetics, personal care and other industries (USD Billion) 33.
One of the regions in the silicone sector with the greatest growth is Asia Pacific and India. The region’s rapidly rising standard of living, higher GDP growth rates, increased use of personal
care and cosmetic products, more sophisticated agricultural farming methods, the shift to electric vehicles where silicone rubbers can meet demanding needs, and increased consumption of textiles and quality of life-related goods are the main drivers of this growth. All of these factors will result in increased demands for LSRs, performance sealants, and functional silicones. The market is anticipated to increase by 7.5 percent in Asia Pacific and by roughly 15 percent in India.
Silicone cosmetics: Health, environment and sustainability aspect
Health:
Silicones are the most extensively researched compounds which utilised in consumer and commercial goods. The silicone industry promotes the safe and responsible use of silicone products, as well as risk-based weight-of-evidence evaluations all over the world for effective human health decision-making.
Silicone manufacturers have done over 1,000 studies to examine the safety of silicones in terms of employees, customers, the environment, and industrial processes 6, 32 – 34. The findings of this ongoing research and testing show that silicones are safe in a variety of critical applications. Independent experts have proven that silicones and siloxanes, in particular, pose no health danger to humans 6, 32, 35.
Especially, government health officials in Canada adopted a risk-based methodology to assess certain siloxanes and found no evidence of a harm to children or adults. Furthermore, the United
States Cosmetic Ingredient Review (USCIR) and the European Scientific Committee for Consumer Safety (ESCCS) stated that there is no risk to human health in silicon cosmetic applications. The current European Commission regulatory for D5 in a concentration equal to or less than 1% w/w or D6 in a concentration equal to or less than 3% w/w and some of the silicone manufacturing companies like, Elkay Silicones, are already committed to supply the silicone material less than 0.1%. Silicones industries will continue to lead research efforts to increase scientific knowledge of silicone materials used in consumer and industrial applications’
safety for human health and the environment.
Environment:
The silicone industry is dedicated to ethical silicone use and environmental management on a global scale. Through rigorous environmental monitoring systems, the industry continues to assess the science behind their products. In March 2016, members of the SEHSC initiated an environmental monitoring programme, created in collaboration with EPA (Environmental Protection Agency), to analyse levels of D4 in the environment in order to provide environmental monitoring data on one of the fundamental components used to produce silicones, i.e. siloxane D4 5. The program’s data will help the EPA assess D4’s environmental danger by utilising real ambient concentrations rather than computer models to anticipate concentrations. Real-world data is being used by governments throughout the world to guide chemical evaluations. For example, after reviewing the scientific evidence and environmental monitoring findings for D4, Environment Canada found that no limitations or product concentration limits were required for D4 usage in any application. After an impartial panel of specialist toxicologists determined that the siloxane known as D5 poses no damage to the environment now or in the future, Canada’s Environment Minister declared that no regulatory limitations on it are necessary 36.
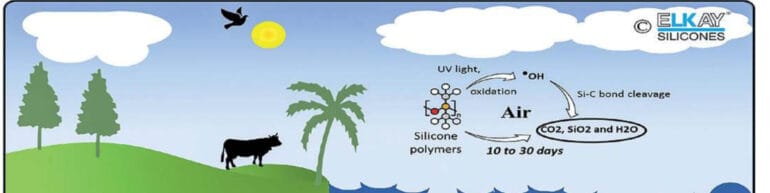
Additionally, several current studies and research are being conducted to better understand the mechanisms of silicone degradation in the environment, as well as any possible ecological
effects on the land, aquatic, and atmospheric compartments 7, 30, 37. In terms of the soil inducement, it was discovered that silicone polymers depolymerize fast due to soil hydrolysis, breaking up siloxane bonds and resulting in lower molecular weight oligomeric siloxane 30, 37. Clay-catalyzed hydrolysis of oligomeric siloxane is a well-researched process for its degradation30, 31. When PDMS is hydrolyzed by clay minerals in soil, dimethylsilanediol is
produced, which is biodegradable to CO2, SiO2, and H2O by microbes Complementary, enzymatic cleavage, it may also depolymerize the chain ends, resulting in a significant drop in the polymer’s molecular weight 39. The rate of this depolymerization via hydrolytic degradation is surprisingly quick and all siloxane linkages were broken within 21 to 30 days, leaving no trace of the silicone polymer 7. Similarly, because of its poor solubility and hydrophobic properties, silicone polymer is unlikely to collect in the aqueous compartment of the atmosphere. Silicone is being transported into the aqueous environment on transit to the wastewater treatment plant. Numerous investigations on the influence of silicone on microbes, invertebrates, amphibians, fish, and other benthic creatures during its temporary stay in the aquatic environment showed no measurable adverse effects. Furthermore, it had no effect on wastewater treatment process parameters (e.g., pH, sludge volume index, suspended solids and settling data, endogeneous
decay, oxygen uptake rates, half-saturation content for substrate utilisation, and any effect on aerobic/anaerobic digestion) or sludge digestion operating parameters 40.
It is also expected that the low molecular weight organosilicons, somewhat volatile organosilicons eventually migrate to the atmospheric and react with the different atmospheric oxidants compartment. However, the lifetime of these compounds is very short, and it can be completely remove from the atmosphere within 10 to 30 days 7,41,42. On a similar note, the impact of volatile organosilicons on air quality, particularly in urban areas, was investigated
to see if these compounds in the lower atmosphere contribute to the formation of tropospheric ozone, smog, or haze, as well as whether they have any other negative effects on air quality. Based on the research study 17, 30, 41–43, it was clearly indicated that even in the presence of other organic compounds, organosilicons do not contribute to the lower atmosphere aerosol
formation of ozone or smog. Also, they have a negative effect on the formation of low-level ozone. Based on these studies the U.S. Environmental Protection Agency excluded organosilicons from its regulation concerning restriction of volatile organic compounds
in the atmosphere.
The investigations and regulatory evaluations listed below demonstrate that silicone materials can be used safely in a range of applications:
- Regulatory bodies in Canada have conducted detailed reviews of scientific evidence and found that silicone products meet regulatory criteria for human health and the environment.
- Health Canada, a federal government organisation, evaluated silicones frequently used in consumer items and came to the conclusion that they are “not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.”
- Considering the entire body of scientific knowledge, according to the Canadian Board of Review “Siloxane D5 does not pose a danger to the environment. There is no evidence to demonstrate that Siloxane D5 is toxic to any organism.” The Board also states that Siloxane D5 “will not accumulate to sufficiently great concentrations to cause adverse effects in organisms in air, water, soils or sediments.”
- According to the EU’s Scientific Committee on Consumer Safety (SCCS), silicones used in cosmetic items don’t endanger human health.
- The Cosmetic Ingredient Review (CIR), an expert scientific group, has determined that silicones do not present a risk to human health from their use in cosmetics.
However, it is speciously believed that silicone cosmetic products being used may have some harmful effects on the health of living beings and they may also cause damage to the environment7.
Thus, argued appeals to continuously monitor silicones in the environment are justified.
Hence, some voluntary initiatives plan the research program to monitor the silicone accumulation and distribution in environment, aquatic biota and human. Thus, in 2010, the silicones industry established a Monitoring Program for D4 and D5 as part of its commitment to product stewardship. The Silicones Monitoring Program is conducted in four areas throughout the world to offer a representative view of silicone materials destiny and distribution:
Lake Pepin in the United States, Lake Ontario in Canada, Osloforjd in Norway, and Tokyo Bay in Japan.
Such kind of research studies and state-of-the-art modeling techniques will definitely aid in the understanding of silicone exposure in the environment, which will be useful to both the silicone
industry as well as humanity.
Sustainability:
Silicones play a vital role in encouraging sustainable development since they improve the performance and durability of a wide range of commercial and industrial products. Additionally,
silicones help in the application of sustainable energy sources such as wind and solar power. The mechanical and chemical characteristics of silicone encapsulation in solar panels contribute to their long-lasting use and lower repair costs. The durability and weather resistance of the rotor blades of wind turbines can be improved by using silicone bonding agents and lubricants during production. Since silicone lends its beneficial features to other materials, products made with silicone use less energy and resources and last longer, spread better, remain flexible or stiff, and can withstand extreme temperatures or humidity.
Due to their extremely low use rates and superior efficiency, silicones are a greener alternative to frequently used petroleumbased products. Silicones are increasingly being employed in the
oil and gas business to better collect, transport, and process petroleum, enabling us to make the most effective use of this non-renewable resource, as humans struggle to create other energy
and chemical sources. Animals can be protected with silicones.
Otters caught in a significant oil spill in Arctic seas were treated with PDMS as one of its principal applications. A PDMS coating provided their coat with the essential protection from the icy waters until their natural waterproofing system could recover after the oil had been removed using detergents. Otherwise, they would not have survived.
The benefits of using silicones, siloxanes, and silanes far outweigh the negative effects of manufacture and end-of-life disposal by a factor of nine in terms of energy savings and greenhouse gas (GHG) emission reductions (per tonne of CO2 emitted). The use of silicone chemistry products in the U.S., Europe and Japan yields GHG emission reductions equivalent to about 54 million tons of CO25. In a variety of markets, including those for consumer goods, electronics, transportation, and energy, silicones assist in making items more environmentally friendly.
Conclusion
The personal care market rapidly accepting silicone polymers as a promising ingredient. In fact, 50% of new personal care products contain at least one type of silicone. The ability to achieve
precise qualities, desirable formulation and excellent benefits is what spurs the growth of silicone in cosmetics. From the environmental point of view, due to the superficial use of silicone in cosmetic applications, it might reach municipal wastewater treatment plants. However, based on several studies and research it was concluded that silicone splits onto the sludge and has no
negative effects on the treatment plant since it is so insoluble in water. Subsequently, sludge is burned, buried in a landfill, or applied as fertiliser to fields of crops. Furthermore, silicones permeate the soil ecosystem and are degraded by soil hydrolysis. The volatile silicone components in the upper atmosphere, where hydroxyl radicals break the Si–C bonds, release silica, water, and carbonyl compounds, which are part of our ecosystem. Also, the rate of silicone degradation is remarkably rapid. The volatile silicone degrades in 10–30 days, while hydrolytic degradation in dry soil takes 4–7 days. It implies that silicone polymers or their breakdown products do not accumulate in the environment and not harmful to land or aquatic life or plants.
Needless to say, there is surprising potential of silicone in cosmetics as well as other areas such as healthcare, aerospace, electronics, transportation, construction and energy. However, it is
essential that all regulatory governing authorities and monitoring entities express their concerns and offer the green signal to the future of silicones industries.
References
- N.R. Thomas, Frederic Stanley Kipping-Pioneer in Silicon Chemistry: His Life & Legacy, Silicon. 2 (2011) 187–193. https://doi.org/10.1007/s12633-010-9051-x.
- A.J. O’Lenick, Silicones for Personal Care, 2nd Edition, 2008.
- G. Chandra, L.D. Maxim, T. Sawano, The Silicone Industry and its Environmental Impact, 3 (1997) 295–319. https://doi.org/10.1007/978-3-540-68331-5_12.
- A. Olejnik, B. Sztorch, D. Brz¸akalski, R.E. Przekop, Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives, Materials (Basel). 15 (2022) 1–18. https://doi.org/10.3390/ma15031126.
- GSC, Safety – Silicones Benefits, Https://Siliconesbenefits.Cn/Safety/. (2022). https://siliconesbenefits.cn/safety/ (accessed August 26, 2022).
- K. Mojsiewicz-Pienkowska, M. Jamrógiewicz, K. Szymkowska, D. Krenczkowska,Direct human contact with siloxanes (silicones) – safety or risk part Characteristics of siloxanes (silicones), Front. Pharmacol. 7 (2016) 1–8. https://doi.org/10.3389/fphar.2016.00132.
- D. Graiver, K.W. Farminer, R. Narayan, A Review of the Fate and Effects of Silicones in the Environment, J. Polym. Environ. 11 (2003) 129–136. https://doi.org/10.1023/A:1026056129717.
- R. Chakraborty, Development of Novel Cycloaliphatic Siloxanes for Thermal and UV-Curable Applications, (2008) 1–200.
- Z. Hu, M. Liao, Y. Chen, Y. Cai, L. Meng, Y. Liu, N. Lv, Z. Liu, W. Yuan, A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone, Int. J. Nanomedicine. 7 (2012) 5719–5724. https://doi.org/10.2147/IJN.S37277.
- D. Krenczkowska, K. Mojsiewicz-Pie´nkowska, B. Wielgomas, D. Bazar, Z. Jankowski, Ex vivo human skin is not a barrier for cyclic siloxanes (Cyclic silicones): Evidence of diffusion, bioaccumulation, and risk of dermal absorption using a new validated gc-fid procedure, Pharmaceutics. 12 (2020) 1–23. https://doi.org/10.3390/pharmaceutics12060586.
- P.J. Flory, Statistical Mechanics of Chain Molecules, Phys. Today. 23 (1970) https://doi.org/10.1063/1.3022125.
- F.W. Pietrusza, L.H. Sommer, F.C. Whitmore, A New Synthesis of Organosilicon Compounds, J. Am. Chem. Soc. 70 (1948) 484–486. https://doi.org/10.1021/ja01182a014.
- B.K. Hostettman, K. Hostettman, G.R. Waller, Saponins: Chemistry and Pharmacology of Natural Products, J. Am. Chem. Soc. 118 (1996) 8509–8510.
- N.K. Penta, P.R. Dandu Veera, S. V. Babu, Role of poly(diallyldimethylammonium
chloride) in selective polishing of polysilicon over silicon dioxide and silicon nitride films, Langmuir. 27 (2011) 3502–3510. https://doi.org/10.1021/la104257k. - T. Kunze, M. Posselt, S. Gemming, G. Seifert, A.R. Konicek, R.W. Carpick, L. Pastewka, M. Moseler, Wear, plasticity, and rehybridization in tetrahedral amorphous carbon, Tribol. Lett. 53 (2014) 119–126. https://doi.org/10.1007/s11249-013-0250-7.
- B. Yi, S. Wang, C. Hou, X. Huang, J. Cui, X. Yao, Dynamic siloxane materials: From molecular engineering to emerging applications, Chem. Eng. J. 405 (2021). https://doi.org/10.1016/j.cej.2020.127023.
- Y. Lin, F. Yin, Y. Liu, L. Wang, Y. Zhao, M. Farzaneh, Effect of ultraviolet- A radiation on surface structure, thermal, and mechanical and electrical properties of liquid silicone rubber, J. Appl. Polym. Sci. 136 (2019) 1–11. https://doi.org/10.1002/app.47652.
- M.S. Kalairaj, T.Z. Feng, H. Ren, Soft-bodied flexible bending mechanism with silent shape memory alloys aiming for robotic endoscopy, INC, 2020. https://doi.org/10.1016/B978-0-12-817595-8.00010-9.
- M. Andriot, S. Chao, A. Colas, S. Cray, F. DeBuyl, J. DeGroot, A. Dupont, T. Easton, J. Garaud, E. Gerlach, Silicones in Industrial Applications, Inorg. Polym. 84 (2009) 61–161.
- A. EL-Hamouz, M. Cooke, A. Kowalski, P. Sharratt, Dispersion of silicone oil in water surfactant solution: Effect of impeller speed, oil viscosity and addition point on drop size distribution, Chem. Eng. Process. Process Intensif. 48 (2009) 633–642. https://doi.org/10.1016/j.cep.2008.07.008.
- Z. Farhadinejad, M. Ehsani, I. Ahmadi-Joneidi, A. Shayegani, H. Mohseni, Effects of UVC radiation on thermal, electrical and morphological behavior of silicone rubber insulators, IEEE Trans. Dielectr. Electr. Insul. 19 (2012) 1740–1749. https://doi.org/10.1109/TDEI.2012.6311523.
- D.C.C. França, A.L. de Castro, A.M.P. Soubhia, S.M.H.C.Á. de Aguiar, M.C. Goiato, Evaluation of the biocompatibility of silicone gel implants – histomorphometric study, Acta Inform. Medica. 21 (2013) 93–97. https://doi.org/10.5455/aim.2013.21.93-97.
- M. Zare, E.R. Ghomi, P.D. Venkatraman, S. Ramakrishna, Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies, J. Appl. Polym. Sci. (2021) 1–18. https://doi.org/10.1002/app.50969.
- J. Woodruff, Silicones and silicone alternatives 1st Published in SPC – 2015 Silicones and silicone alternatives 1st Published in SPC – 2015 John Woodruff, (2015).