Abstract
Hair loss is a common occurrence for both women and men, and a major cosmetic concern. Consumer concerns over the side effects of drugs such as Minoxidil© and Finasteride©, have given rise to numerous cosmetic developments in order to address these concerns. In this preliminary investigation, we present our initial findings, and demonstrate beneficial effects of a curcumin-niacinamide formula on the reduction of hair loss, and the improvement in hair density, when applied topically to the scalp. In this 5-month clinical study, hair growth and hair loss evaluation was quantitatively and objectively measured with TrichoScan© analysis, and image photography.
Volunteer subjective assessments, of the effect of the test product by self-perception of reduced hair loss, increased number of new hairs, as well as issues relating to the tolerance and acceptance of the product, were also evaluated.
Introduction
The human hair follicles are very important skin appendages, and play a unique role in skin function and renewal1. A unique “micro-organ”, the hair follicle is formed by the interaction between the skin’s epidermis and dermis, and is composed of complex multi-component microstructures, all playing significant roles in hair growth and condition. Structurally, the hair follicle is rich in stem cells which are regulated by a variety of signalling pathways, and are important for the growth of hair as well as playing an important contribution to skin renewal following injury.
Hair follicles have a high capacity for renewal, and possess a defined periodic growth cycle that continues during human lifetime.
Hair growth is affected by many factors including age, the environment, diet, and general health2. Such factors impact the development of a variety of hair follicle disorders including hair loss (alopecia).
Mainly seen in adults, alopecia or hair loss is a common disorder3. Androgenetic alopecia (AGA, also called male and
female pattern alopecia or baldness) is hormone driven and the most common cause of hair loss4. Depending on age and ethnicity, it is estimated to affect 30 – 58 % of men by the age 50 and 12 – 40 % of women4, 5.
Regulation of the hair cycle is multi-complex and involves an incompletely understood interplay between endocrine, autocrine and paracrine signalling pathways. Hair loss is characterised by the progressive “miniaturisation” of the follicles, resulting from the actions of androgen hormones on the epithelial cells of genetically susceptible hair follicles in androgen-dependent areas 5. The hair cycle consists of three phases: the growth phase, which is called anagen, the resting phase, which is called catagen, and the shedding phase, which is the telogen phase. Approximately ninety percent of hairs are in the growth phase (anagen) and the remainder,
correspond to ten percent in the transition (catagen) and shedding (telogen) phases 5.
While complex genetic inheritance and age of the individual are major risk factors in AGA development, inflammation at the cellular level within the follicle is central to the hair loss process, as well as the effects of abnormal signal transduction (the wingless-type integration site pathway), high levels of apoptosis, and oxidative stress 6. Cosmetically, hair loss can be exacerbated by over-use of hair dyes, hair straightening and styling products, as well as improper use of shampoos and conditioners. The management of hair loss normally takes a pharmaceutical approach with drugs such as Minoxidil® (anti-hypertensive drug) and
Finasteride®, a 5-alpha-reductase inhibitor 7, 8. In the field of cosmetics, interest in hair loss reduction especially those formulated with natural products or their extracts has increased. As mentioned previously, since multiple factors contribute to hair loss, its management requires a multi-action approach. For example, vitamins and trace minerals are vital to the hair follicle cycle9, and help maintain homeostasis as enzyme cofactors, hormones, antioxidants, and immuno-modulators, etc.
Widely used in cosmetics 12, the genus Curcuma of the family Zingiberaceae has been important for its wide variety of benefits since ancient times, and many species of Curcuma have been
identified, with Curcuma longa having been the most intensively scientific researched 10–12. The rhizome Curcuma longa L. (Zingiberaceae) commonly known as Turmeric, is reported as safe 11,
and has been, by certain literature, reported to have a multitude of biochemical activities notably those reducing inflammation, and the reduction of oxidative stress 13, both key features in the
hair loss process. Curcumin, the active ingredient of turmeric, has been used for centuries, and literature reports its ability to down regulate cyclooxygenase-2, lipoxygenase, and inducible nitric oxide synthase enzymes 14, and potentially inhibits nuclear factor-kB signalling, thereby decreasing pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1 15. Additionally, TNF-α and IL-1 are involved in follicular regression 16.
Curcumin is also reported in the literature to have antioxidant, antimicrobial, anti-neoplastic, and anti-androgenic properties 17.
Niacinamide is the amide derivative of Vitamin B3 (niacin). Its action on the skin has been well reported including antipruritic, sebum regulating, and vasoactive effects 18. It has also been reported that niacinamide could enhance hair growth by preventing oxidative stress-induced cell senescence and premature catagen entry of hair follicles 19. Furthermore, reports indicate that niacinamide controls the NFκB-mediated transcription of signalling molecules 20 by inhibiting the nuclear poly (ADP-ribose) polymerase-1 (PARP-1). Given its “flushing” effect on the skin due to its vasoactive properties, niacinamide is a well-tolerated and safe substance used in a wide variety of cosmetic applications 21, 22.
Based on information in published reports, the primary objectives of this preliminary investigation were to ascertain whether a curcumin and niacinamide based serum (Bio-Pilixin®) could help reduce hair loss when topically applied over a period of 5 months, to the scalp as measured quantitatively and objectively by hair density Trichosan® analysis and image photography 23. Volunteer subjective assessments of the effect of the serum under study by
self-perception of reduced hair loss, increased number of new hairs, as well as issues relating to the tolerance and acceptance of the product were also evaluated 23.
Methods
1. Volunteers
This was a single centre, 150-day study (Capillary Technology Centre (CTC), Barcelona, Spain). Thirty (30) healthy male and female volunteers with a mean age of 47± 11 years, were recruited
onto the study in accordance with the principles of Good Clinical Practice (GCP). All volunteers signed a consent form and agreed to follow instructions. Assessments for the type of hair loss experienced by the volunteers was recorded as follows:
| Volunteers | Mean Age (Years) | Hormonal Hair Loss | Seasonal Hair Loss | Stress hair Loss |
| Male | 49 | 3 | 1 | 0 |
| Female | 47 | 11 | 9 | 6 |
2. Inclusion/Exclusion Criteria
Healthy male and female adults, willing to sign an informed consent and motivated to commit to the study timeline, aged between 18–65 years exhibiting hair loss, were eligible to enrol for the study. Volunteers with cardiac and psychiatric disorders, and other medical conditions likely to interfere with the study outcomes, were excluded from the study. Volunteers with known
sensitivity to any of the ingredients in the formulation, and those with scalp diseases such as psoriasis, dermatitis and eczema were also excluded. Volunteers taking medications known to cause alopecia such as fluoxetine, retinoids, and anti-coagulations were excluded from the study, as well as volunteers taking nutraceutical products for reducing hair loss. Volunteers modifying their diet during the course of the study would be excluded.
3. Study Schedule
(i) Test Product – A serum-based product (Bio-Pilixin® Serum, Scandinavian Biolabs) comprising: (INCI) Aqua, Alcohol, Niacinamide, Caffeine, Curcuma Longa Callus Conditioned Media, Panthenol, Vanillyl Butyl Ether, Sodium PCA, Sodium Lactate, Arginine, Aspartic Acid, PCA, Pentylene Glycol, Zinc PCA, Glycine, Alanine, Serine, Valine, Isoleucine, Proline, Threonine, Histidine, Phenylalanine, Phytic Acid, Parfum.
(ii) Product Application – Volunteers applied the test product daily (ca. 4 ml) at night time on a clean scalp, according to instructions.
A minimum of 2 pipettes was considered enough to cover the entire scalp. The product was to be gently massaged into the scalp, paying attention to hair roots. Volunteers were also provided with a neutral shampoo to use on alternate days to prevent any subsequent interpretation bias of data.
(iii) Schedule – 48 hours prior to study commencement (T-2), volunteers were requested not to wash their hair, and not to comb their hair (T0) on the day of study centre visit.
The clinical schedule was as follows:
| Measurement | T0 | T45 | T150 |
| Global Photography | ✓ | ✗ | ✓ |
| Trichogram | ✓ | ✗ | ✓ |
| Hair Density | ✓ | ✗ | ✓ |
| Comb Test | ✓ | ✓ | ✓ |
| Wash Test | ✓ | ✓ | ✓ |
| Volunteer Questionnaire | ✓ | ✓ | ✓ |
4. Measurements
(i) Photography – Standardised global photography, using a Canon EOS 600D digital camera, of the mid-scalp sagittal area was used to capture standardized images of volunteers. Parting the scalp hair was achieved with the aid of a cotton-tipped applicator, aligning the centre of the hair part with the patient’s nasal bridge. Photographs were taken at T0 and T150 and then compared.
(ii) Trichograms (Hair Removal) – This consisted of extraction of a determined number of hairs from the left parieto-occipital area of the scalp on days T0 and T150, by traction plucking with tweezers in the direction of hair growth to minimise damage. As a precondition, volunteers were instructed not wash nor treat their hair with any cosmetic product at least 48 hours before the cosmetic trichogram was carried out, in order to retain any hairs which were near the end of the telogen phase and to avoid artificial reduction in the percentage of telogenic hairs observed in the trichogram. A fixed area is marked on the scalp through a template with a uniform pen.
Plucking was repeated as many times as necessary to obtain the number of hairs required for the cosmetic trichogram, and hair bulbs were immediately mounted onto glass slides for micro-
scopic evaluation – in order to identify which phase of the their growth cycle these hairs were present – anagen growth phase or catagen/telogen fall phase, as well as the assessment of both initial and final appearance of the hair bulbs.
Results were expressed accordingly:
A0 Number of hairs in anagen phase at T0 (before product application).
T0 Number of hairs in telogen phase at T0 (before product application).
%A0 Percentage of hairs in anagen phase at T0 (before product application).
%T0 Percentage of hairs in telogen phase at T0 (before product application).
A150 Number of hairs in anagen phase at T150 (after 150 days product application).
T150 Number of hairs in telogen phase at T150 (after 150 days product application).
%A150 Percentage of hairs in anagen phase at T150 (after 150 days product application).
%T150 Percentage of hairs in telogen phase at T150 (after 150 days product application).
(iii) Hair Density – The number of hairs present per unit of area (cm2) was calculated from TrichoScan® (TrichoScan, Tricholog GmbH, Germany), phototrichogram micro-camera images (Dino-Lite Pro Digital Microscope (micro-camera), Naarden, Holland), of the scalp (40× magnification and reported as units/cm2). TrichoScan® image analysis software permitted counting of the number of hairs present in an area equaling 0.592 cm2 of scalp. For comparison between the number of hairs present at the end (T150) and at the start (T0) of the study, the increase in the number of hairs in that area was recorded and hair density calculated at the end of the study (T150).
(iv) Hair Root Bulb Imaging – With a light microscope, standardised photomicrographs of plucked hair roots taken at the beginning (T0) and at the end (T150) of the study, of the three most representative results for each study group, were evaluated to correlate findings of the trichograms, and to assess the condition of the hair bulbs pre- and post-application (T0) and (T150).
(v) Cape Combing Test – Using a standardised comb test, the number of hairs falling out under test conditions was determined on days T0 and T150. As a precondition, volunteers came to the study centre without having washed their hair at least 48 hours prior to study commencement, and without having combed their hair at least 24 hours, in order to assess hairs near the end of the telogen phase, and to avoid artificial reduction in the percentage of hairs in telogen phase. Falling hair – in the comb and on the cape – was collected for counting.
(vi) Wash Test – The number of hairs falling out during hair washing under standardized conditions was determined. As a precondition, volunteers came to the study centre without having washed their hair at least 48 hours prior to study commencement, and without having combed their hair at least 24 hours, in order to assess hairs near the end of telogen phase and avoid artificial reduction in the percentage of hairs in telogen phase. Any hair from the sink basin was then collected for counting.
5. Volunteer Subjective Assessments
Volunteers evaluated, via an 18 question response form, a variety of aspects of their hair at 3 control points (T0, T45 and T150). Responses were recorded and data evaluated.
6. Statistics and Data Analysis
Statistical descriptive analysis was performed for each biometric quantitative variable along experimental times, including basic descriptive parameters (central tendency and variance) that define the distribution for each response variable in test product and control along experimental times. Linear mixed-effects models were adjusted to data distributions for each response variable, in order to assess the application efficacy of the tested products versus control along experimental times. The application effect of principal variables was interpreted by comparing test product to control in baseline and each experimental time. Every model used in this study for data analysis is contained in the lme function of nlme package for R software. In order to evaluate the efficacy between applications at each time point, normally distributed data that could not be fitted by a linear mixed-effects model were analysed using a paired Student’s t-test. The effect of test product on biometric measures was interpreted by comparing applications with each other at each time point. In order to compare timepoints with baseline for each application, normally distributed data that could not be fitted by a linear mixed-effects model were also analysed using a paired Student’s t-test. The effect of time on biometric measures was interpreted by comparing timepoints with baseline for each application.
In cases of non-normality of data distribution, a Wilcoxon Signed-Rank test was performed for comparisons between test product and control in baseline and each experimental time. In addition, the experimental times relative to baseline are compared marginally for test product and control. Where the null hypothesis of no differences amongst test product and control in each time point was rejected, it can be concluded that there are significant differences amongst test product and control in that experimental time. Where the null hypothesis of no differences among each experimental time and baseline were rejected, it can be concluded that there are significant differences among the time point under assessment and baseline.
Results
All volunteers completed the study with no drop-outs, and no adverse events were recorded.
(i) General Photography – Figure 1 is representative of the three most representative general differences seen in hair growth patterns observed between T0 and T150. When the product application manages to increase the number of hairs, general photography of the scalp is a simple test that demonstrates product success. In this study, general differences were observed between T0 and T150. The example images shown for volunteers A, and C, a subjective difference, where the appearance of new hair is observed after 150 days of application in the parting areas compared T0. In addition, in volunteer B a subjective increase in hair density is observed in the same part of the hair after 150 days of application.
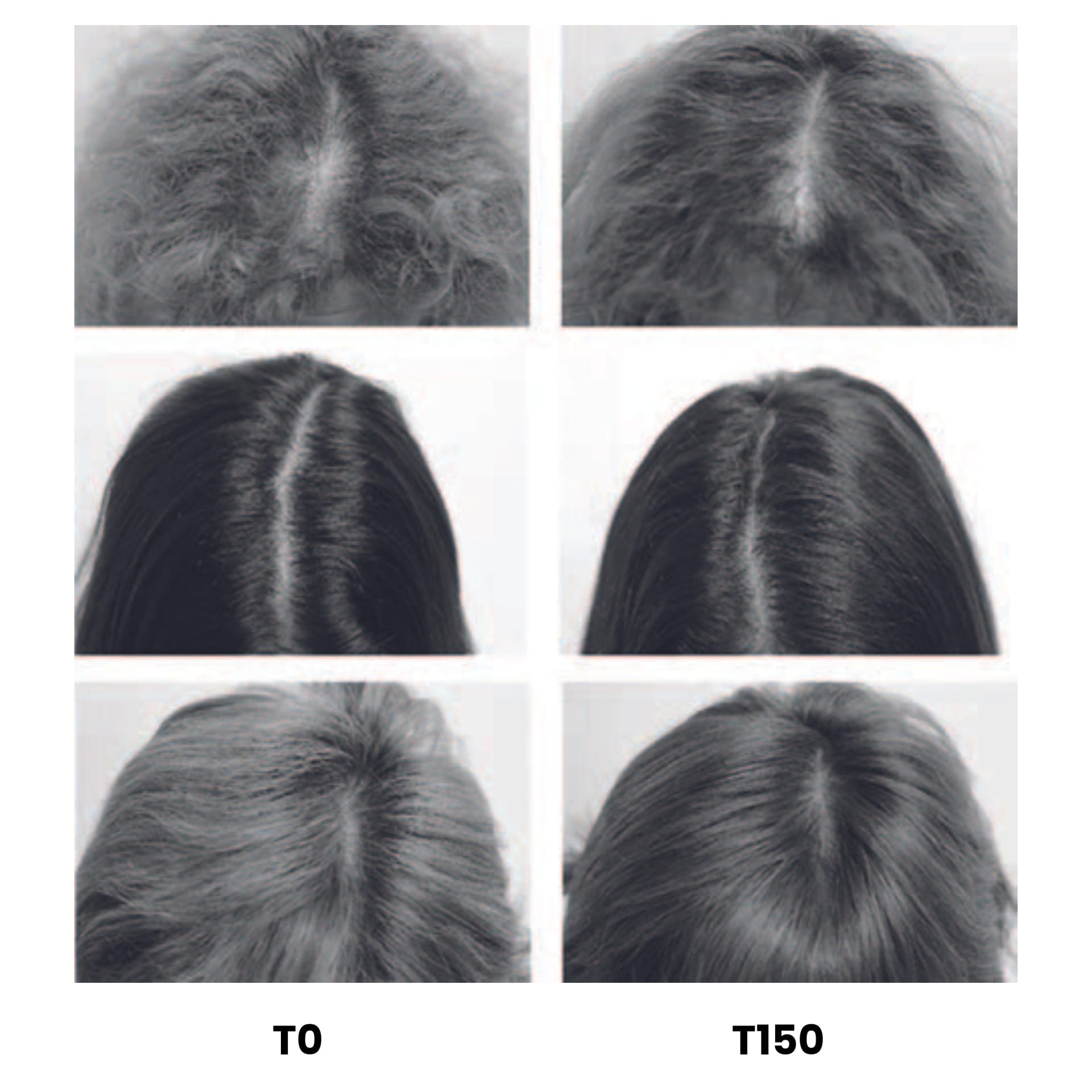
Figure 1: Standardised global photographic (Canon EOS 600D) images of representative (3) volunteers showing subjective changes in hair density, and the subjective appearance of new hairs, following application of test product comparing T0 versus T150 (see text for details).
(ii) Hair Density – As represented in Figure 2 (volunteers D, E, F), observations following 150 days of test product application, showed that 73.3% all volunteers exhibited a higher capillary density. Evaluating the data obtained at T150, the test product was able to increase capillary density with an average value of 5.28%. Taking into account only those volunteers with higher capillary density at T150, the test product was able to increase hair capillary density by an average of 8.64%. At T150 days after product application (Figure 2), capillary density increased at an average of 5% relative to baseline (T0), and all data was statistically significant (p <0.05).

Figure 2: TrichoScan imaging of scalp 3 representative volunteers (D,E.F) showing a quantitative improvement in hair density following 150 days treatment with test product, versus baseline (T0) p < 0.05 (see text for details).
(iii) Effect on Anagen and Telogen Phases – Table 1 shows the percentage of volunteers for whom the test product has had a positive effect (anagen increase and telogen decrease). It was observed that the test product resulted in a positive effect in 90.0% of volunteers with an increase in the anagen phase, and 87% of volunteers showed a positive effect with a reduction in the telogen phase. This is indicative of the test product being able to prolong the growth phase (anagen) of the hair and/or reducing the time hair spent in the telogen phase. By calculating the average values of the increase and/or decrease of the anagen and telogen phases a 17.5% increase in the anagen phase and a 36.5% decrease in the telogen phase (data not shown) was observed. In respect of only those volunteers with an increased anagen phase or decreased telogen phase, there was a 20.03% increase in the hairs in anagen phase and a 43.67% decrease in hairs in the telogen phase. After 150 days of test product application, the anagen phase was increased by 16% relative to baseline (T0), and all data was statistically significant (p<0.05). After 150 days of test product application the telogen phase was decreased by 39% relative to baseline (T0), and all data was statistically significant (p<0.05).
(iv) Effect on Hair Regeneration – Any hair loss reducing product is required to increase the percentage of hairs within the anagen phase as well as decrease hairs within the telogen phase, and thus an increase in the anagen/telogen (A/T) ratio. In this study, it was observed that 90.0% of the volunteers showed an increase in the A/T ratio after 150 days of product application (Table 1). Furthermore the ratio of A/T was increased by 135% relative to baseline (T0), and all data was statistically significant (p<0.05).
(v) Hair Root Bulb Imaging – Plucked hair root bulbs were examined for integrity at T0 and T150 (Figure 3). In the example volunteers (T0) poorly developed or irregular root structure was observed under light microscopy, with a clear telogen phase, and little or no presence of the hair sheath (G and H). In volunteer I, the hair sheath was also absent with an irregular poorly developed root and poorly compacted cohesive keratins. Following application with the test product (T150), the hair roots of volunteers exhibited a well-developed hair sheath and increased thickness corresponding to the anagen phase. Good follicle adherence was also observed.
(iv) Comb and Wash Tests – As illustrated in Table 2, in the combined comb and wash tests, following 45 days of test product, 80.0% of the volunteers exhibited a reduction in hair loss. Of those volunteers exhibiting reduced hair loss, the average actual numbers of hairs was reduced by 13.9%. After 150 days of application 93% of the volunteers exhibited a reduction in hair loss, and of those volunteers exhibiting reduced hair loss, the actual average reduction of hair numbers lost was increased to 28%. At 45 days after application, the numbers of hairs lost decreased was 17% relative to T0, while at T150, the number of hairs lost decreased by 50% relative to T0. These differences were statistically significant (p<0.05).
(v) Volunteer Subjective Assessments – A pre-study questionnaire was completed by all volunteers with regard to insights on their hair loss concerns, scalp condition, and general hair condition (Table 3). All volunteers exhibited hair loss with 90% rating that loss as either moderate (50%) or severe (40%). The hair lost was considered either thick (33.3%), thin (46.7) or both (20%). Of interest, while to majority of volunteers exhibited little (30%) or no dandruff (56.7%), the majority of volunteers (93%) indicated they had an itchy scalp. The reasons are unclear, though the majority of volunteers were women (26), and the number of volunteers who dyed their hair was 63%.
During the course of product usage, volunteer subjective assessments correlated with clinical objective evaluations in that a reduction of hair loss was seen. 77% of volunteers indicated a hair loss reduction at T45 which increased to nearly 97% at T150 (Figure 4). Clinical observations of hair density also correlated with volunteer assessment, with volunteers indicating an increase in hair density, of 17% and 30% at T45 and T150 respectively. Approximately 47% of volunteers felt a slight increase – correlating with the data that around approximately 77% of volunteers in fact had an increase in hair density based on the Trichoscans. Furthermore, volunteers also noted a good reduction (intense and moderate improvements) in hair loss after combing and washing, notable after 150 days application (67% and 70% respectively). Considering whether the product could be considered as having an actual hair loss reducing effect, 53% agreed (intense and moderate agreement) with 40% agreeing to the product having a slight benefit. Other observations (data not shown) indicated that 97% of volunteers felt the product caused no scalp discomfort; 67% of volunteers said they noticed new hairs, and 95% scored this notice as either intermediate (75%), or higher (20%).
Discussion & Conclusions
In this preliminary study we have been able to show, that when combined with niacinamide18, 19, the Curcumin longa and niacinamide led cosmetic formula was able to help reduce the loss of hair in human volunteers over a 5 month test period23. Standard methods employed to evaluate the cosmetic improvement in hair loss and hair density provided valuable insights into the performance of the product under test. The results obtained correlated well with subjective volunteer assessments, despite the low number of volunteers recruited onto the study, and the imbalance between male and female volunteers. However, in order to solidify the data, a larger cosmetic study is in planned process, in terms of volunteer numbers, with a more balanced male-female ratio, the type of hair loss, and age, etc. Furthermore such a larger study will enable us to focus on the hair loss parameter between the sexes, as well as validate the data presented herein.
While clinical quantitative methods, including hair loss and pattern type, as well as clinical grading of severity are commonly used and valuable in demonstrating the effectiveness of any cosmetic hair loss product, unless potential consumers can see the effects for themselves, photographic imaging and volunteer subjective questionnaires with respect to cosmetic product performance are vital. Moreover, a better understanding of the “individual” in terms of their nutritional status, hormones, environmental exposure, stress, use of hair dyes, straighteners, bleaching and the like, etc., are also required.
The hair loss phenomenon is distressing for those individuals suffering from the condition, and self-perception of image and loss of attractiveness is still poorly understood and poorly addressed by the cosmetic industry. This could partly be due to legislation classifying cosmetic hair loss/hair loss reducing products, such as the one studied here, as “borderline”24, whereby each product is assessed individually on a case-to-case basis, with few clear guidelines for the industry. This could potentially make it harder to engage the community in developing, testing and improving such products.
Acknowledgements
The authors would like to thank Dr Theresa Callaghan, Callaghan Consulting International Germany, for the preparation of this manuscript and fruitful discussions.
References
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic mini-organ. Curr. Biol. (2009),19:R132-42. doi: 10.1016/j.cub.2008.12.005
- Lin X, Zhu L, He J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. (2022), 10:899095. doi: 10.3389/fcell.2022.899095
- Llamas-Molina J, Carrero-Castaño A, Ruiz-Villaverde R, Campos A. Tissue engineering and regeneration of the human hair follicle in androgenetic alopecia: Literature review. Life (Basel), (2022), 12:117. doi: 10.3390/life12010117
- Lolli F, Pallotti F, Rossi A. et al. Androgenetic alopecia: A review. Endocrine (2017), 57:9-17
- Bertoli M, Sadoughifar R, Schwartz R et al. Female pattern hair loss: A comprehensive review. Dermatol Ther. (2020), 33:e14055. doi: 10.1111/dth.14055
- Trüeb, R. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. (2021), 43, S9–S13
- Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des. Devel. Ther. (2019), 13:2777-2786
- Irwig M. Persistent sexual and non-sexual adverse effects of Finasteride in younger men. Sex Med Rev. (2014), 2:24-35
- Almohanna H, Ahmed A, Tsatalis J, Tosti A. The role of vitamins and minerals in hair loss: A review. Dermatol Ther (Heidelb) (2019), 9:51-70. doi: 10.1007/s13555-018-0278-6
- Goncalves G, da Silva G, Barros P. et al. Use of Curcuma longa in cosmetics: extraction of curcumoid pigments, development of formulations, and in vitro permeation studies. Braz. J. Pharm.Sci. (2014), 50:4. https://doi.org/10.1590 S1984-82502014000400024
- Ahmad R, Hussain M, Sultan M. et al. Biochemistry, safety, pharmacological activities, and clinical applications of Turmeric: A mechanistic review. Evidence-Based Complementary and Alternative Medicine Volume 2020, https://doi.org/10.1155/2020/7656919
- Gopinath H, Karthikeyan K. Turmeric: A condiment, cosmetic and cure. Indian J. Dermatol. Venereol. Leprol. (2018), 84:16-21
- Peng Y, Ao M, Dong B, Jiang Y. et al. Anti-inflammatory effects of Curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Devel. Ther. (2021), 15:4503-4525
- Rahaman M, Rakib A, Mitra S, Tareq A. et al. The genus Curcuma and inflammation: Overview of the pharmacological perspectives. Plants. (2021), 10:63. https://doi.org/10.3390/plants10010063
- Yin H, Guo Q, Li X, Tang T, Li C et al. Curcumin suppresses IL-1b secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. (2018), 200: 2835–2846
- Gorabi A, Razi B, Aslani S, Abbasifard M. et al. Effect of curcumin on pro-inflammatory cytokines: A meta-analysis of randomized controlled trials. Cytokine, (2021), 143: 155541. https://doi.org/10.1016/j.cyto.2021.15554
- Sivani B, Patnaik R, Pantea-Stoian A. et al. Reconnoitering the therapeutic role of Curcumin in disease prevention and treatment: Lessons learnt and future directions. Metabolites (2022), 12: 639. https://doi.org/10.3390/metabo12070639
- Wohlrab J, Kreft D. Niacinamide – mechanisms of action and its topical use in dermatology. Skin Pharmacol. Physiol. (2014), 27: 311-315. doi: 10.1159/000359974
- Choi Y, Shin J, Kim J, Kang N, Lee S. Niacinamide down-regulates the expression of DKK-1 and protects cells from oxidative stress in cultured human dermal papilla cells. Clin. Cosmet. Invest. Dermatol. (2021), 14:1519-1528. doi: 10.2147/CCID.S334145
- Si Y, Zhang Y, Zhao J, Guo S. et al. Niacin inhibits vascular inflammation via down-regulating nuclear transcription factor-kB signalling pathway. Mediators of Inflammation (2014) https://doi.org/10.1155/2014/263786
- Huber R, Wong A. Nicotinamide: An update and review of safety & differences from Niacin. Skin Therapy Lett. (2020), 25: 7-11
- Matts P, Oblong J, Bissett D. A Review of the range of effects of niacinamide in human skin. Int Fed Soc. Cosmet Chem. Mag.(2002), 5: 285-289
- Scandinavian Biolabs Study Report: Clinical efficacy study of the
Bio-pilixin® formula used in a hair serum. Report ID No. ID034-21 February 2022 - Manual on the scope of application of the Cosmetics Regulation (EC)
No 1223/20009 (Art 2(1)(A)). November 2013



