
The popularity and extent of sustainable ingredients into the formulator toolbox continues unabated. Many chemists and the brands they support are probing away from use of ethoxylated (ETO) surfactants and additives given the current climate and the stigma assigned to these PEGylated compounds. With the negative public reaction to occasional ETO releases from medical sterilization plants, government scrutiny of 1,4-dioxane content, and their non-renewable content, the value proposition of any ingredient having “PEG” or “eth” in its INCI name has diminished lately. Though the ethoxylates continue to serve the industry well for over 75 years, alternative materials and methods are now of keen interest.
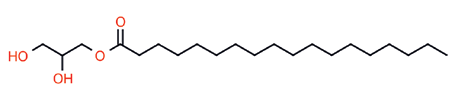
The polyglyceryl esters of fatty acids (PEFA) lend themselves to this cause to offer excellent emulsification tools and more. Although these renewable surfactants have been employed commercially since the 1960’s, very little has been published on their utility and strengths. This knowledge gap and the consideration gap that accompanies it are addressed here. Structurally, the PEFA have their origin in glycerin esters such as glyceryl monostearate (GMS.) The emulsifying compound pairing GMS with a small amount of potassium stearate, introduced by Goldschmidt AG in the 1950’s as Tegin A®, proved to be the most widely used cosmetic cream emulsifier of that era. It was only logical that chemists involved in surfactant syntheses recognized expanding the glyceride hydrophile might lead to useful ester oligomers. This proved to be the case. Esterifying polymeric glycerin (PG) led to many amphiphilic materials having diverse properties all from renewable starting materials.
Manufacturing technology and customization
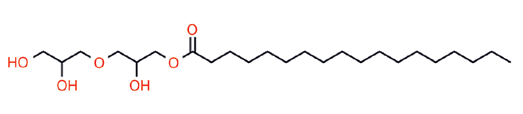
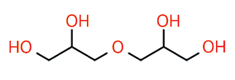
Synthesis of these materials is founded in two steps. Glycerin is dimerized by a condensation reaction into linear diglycerin, which then undergoes asymmetric addition to create larger polyglycerides: PG-2 + PG-2 = PG4, PG-4 + PG2 = PG-6, PG-6 + PG-4 = PG10. These homologs maintain high linearity in this fashion being derived from diglycerin. Addition of mono-glycerin will produce more branched oligomers having less conformational uniformity.2 This is why there are very few commercial Polyglyceryl-5, 7, or 9 oligomers, nor many PG-3. There are 8 possible isomers for polyglycerin-3. Furthermore, beyond polyglyceryl-10 (PG-10) this “goes off the rails” producing dendrimers. PG-10 is therefore the current practical oligomeric limit.
The next step is esterification of the polyglyceride backbone. There are several methods of doing so, most practical being a modified Steglich reaction,8,9 where the fatty acid forms a nucleophilic amido-functional intermediate capable of SN2 substitution of hydroxyls bonded to both primary and secondary glycolic locations. The reaction proceeds under mild conditions and modern methods of Steglich use green solvents. Mild conditions are important in that high temperatures and pressures may lead to enol elimination resulting in olefins.
The esters created allow much versatility for commercial application.
Index #1. Attributes are a function of fatty acid character, polyglyceride oligomer and degree of ester substitution.3,7
- Polyglcyeryl-2 Laurate: plasticizer for aqueous & solvent nail enamels
- Polyglyceryl-3 Diisostearate: pigment dispersant & emulsifier
- Emulsiderm PG4C (Polyglcyeryl-4 Caprate): lipid solubilizer/refatting agent
- Emulsiderm PG4S (Polyglyceryl-4 Stearate): o/w primary emulsifier
- Polyglyceryl-4 Isostearate: co-emulsifier for invert-phase systems
- Emulsiderm PG6L (Polyglyceryl-6 Laurate): o/w emulsifier for liquid mix
- Emulsiderm PG6O (Polyglyceryl-6 Oleate): o/w emulsifier for higher loading
- Polyglyceryl-6 Polyricinoleate: w/o primary emulsifier
- Polyglyceryl-6 Octastearate: crystallization inhibitor for oils, butters
- Polyglyceryl-10 Laurate: solubilizer, hydrotrope, detergent
- Polyglyceryl-10 Dilaurate: 2° emulsifier, detergent
- Polyglyceryl-10 Hexaoleate: corrosion inhibitor, fabric softener
When multiple ester substitutions take place in PEFA, polydiversity ensues. Location of the fatty ester lipophile can vary and the greater degree of substitution on longer the polyglcyeride oligomer, the higher the polydiversity index.3,6 This can be a constraint but also an advantage as multi-esters convey unique properties to PEFA. (See Index #1) Indeed it is this condition that makes these chemical entities so useful in formulation chemistry, an unheralded quality in the author’s opinion.
In formulating with PEFA know there are four basic types:
Discrete (mono) esters: e.g. Polyglcyeryl-4 Laurate, monoester of a discrete fatty acid. Mixed (mono) esters: e.g. Olive Oil Polyglcyeryl-6 Esters, monoester of multiple fatty acids. Discrete multi-esters: e.g. Polyglcyeryl-3 Diisostearate, multiple degrees of esterification with a discrete fatty acid.
Mixed multi-esters: e.g. Polyglcyeryl-4 Diisostearate/Polyhydroxystearate/Sebacate, multiple degrees of esterification by multiple fatty acids.
Character and Safety
Looking at PEFA chemical anatomy the character that stands apart from PEG surfactants is the greater number of hydrogen bond donor hydroxyls, their ratio to hydrogen bond acceptor oxygens and the larger polar surface areas occupied. PEFA share this character with surfactant chemistry based on alkyl-modified saccharides such as the glucosides and rhamnolipids. With the exception of sucrose esters, these compounds tend to work mainly as detergent surfactants in cleansing products, a quality they possess superior to that of PEFA. Yet PEFA afford more functions than any of these compounds, especially in emulsification.
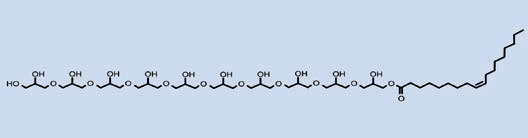
H-bond donor = 11, H-bond acceptor = 22, Polar surface area = 332 Å2
PEFA compounds add many deliverables to the green formulator’s toolbox, not only as o/w emulsifiers but as pigment dispersers, crystallization inhibitors, refatting agents in cleansers, w/o invert co-emulsifiers, micellar water conveyors, niosome vesicle constructors,4 sensorial enhancers and preservative boosters. Their versatility arises from the high degree of customization afforded by the chemistry itself, altering fatty acid moiety (see Index #2), degree of hydroxyl substitution and polyglyceride oligomer. Their chemical cousins the modified saccharides do not share this degree of versatility to the same extent as the PEFA.
What we also know about PEFA is their excellent safety profile as these are well regarded by state regulatory agencies globally. Food technologists have used them for many decades.5 Being nonionic surfactants, the PEFA exhibit compatibility with all types of ingredient chemistry and rheology modifiers. They accommodate lipids of varying polarity, stability with electrolytes and proteins, and offer no interference with preservatives. Thus it can be said that polyglyceryl esters are useful surfactants that play well with others. As a value-added quality PEFA lend pleasant sensorial effects to emulsions. These are esters, after all. While primarily capable as o/w emulsifiers, the PEFA can function as superb w/o emulsifiers and co-emulsifiers with alkyl ether silanes as primary emulsifiers for high internal phase (HIP) invert emulsions. Although many PEFA may emulsify lipids as sole surfactant, in our work we found these compounds work more effectively when used in tandem with each other or with ancillary surfactants as contributors: the buddy blend system as it were.
Experiment and rationalization
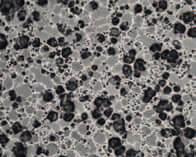
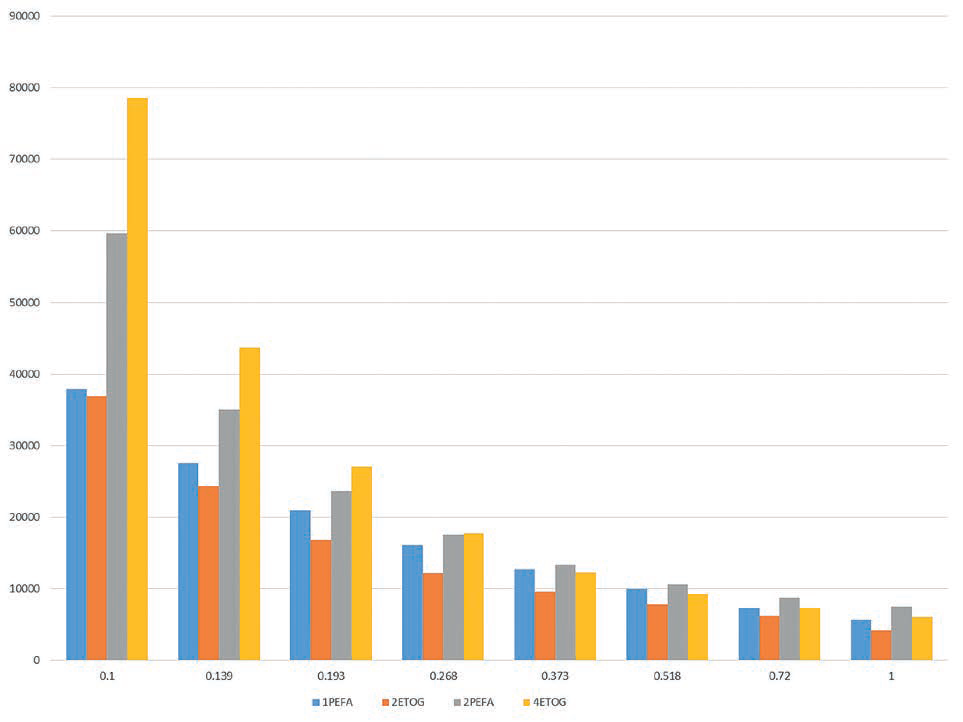
We examined rheological attributes of four oil-in-water lotion formulations all sharing same emulsion chassis and medium polarity lipid concentrations. Processing conditions were held consistent and no rheology stabilizers were employed. Two were based on PEGylated surfactants while other two were based on PEFA in parallel fashion. Compounding the four then subjecting to parallel study on our rheometer and texturometer, we compared attributes. All four were similar in appearance and determined to be stable. Yet, there were differences.
The formula 4ETOG is based on a “classic PEG” Tween™ 60/Span™ 60 emulsifier combination as described in Griffin’s seminal HLB handbook10 of the 1950’s while its tandem formula 2PEFA was based on our Emulsiderm™ PEFA “buddy blend” of same total concentration and predicted HLB as the classic PEG. Photomicrographs of both exhibited smaller net average curvature (NAC) in the droplets formed by the PEFA emulsion with attendant lower viscosity even though stability and outward appearance was virtually same. (see Example 1)
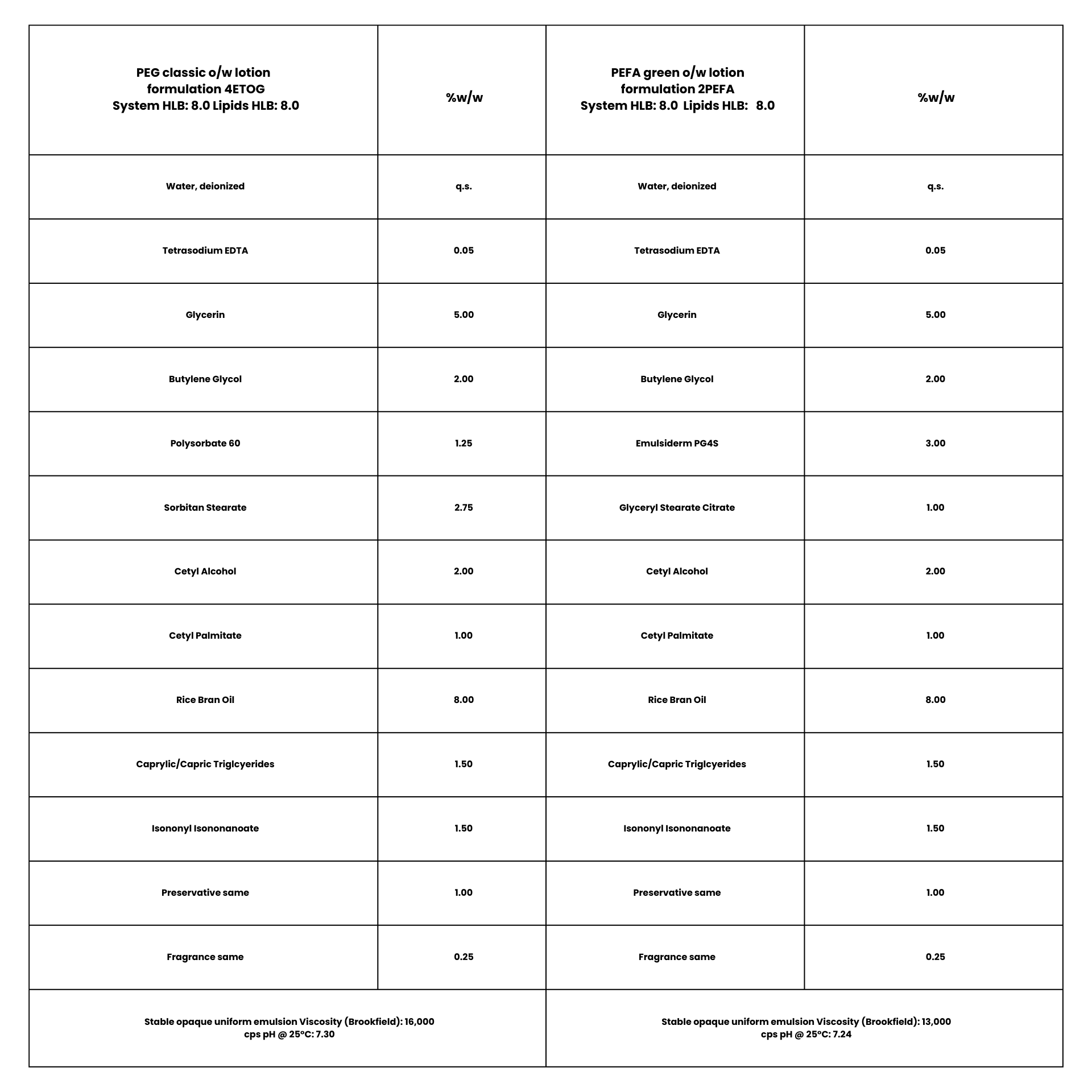

Photomicrograph of
Formulation 4ETOG
Photomicrograph of
Formulation 2PEFA
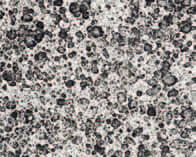
The other two formulations used same co-emulsifier, glyceryl monostearate, but differing primary emulsifiers: PEG-8 C12-18 alkyl ester in the benchmark 2ETOG, polyglcyeryl-6 oleate in the alternate tandem formula 1PEFA . Concentrations and predicted HLB were same in both. This time the PEFA alternative exhibited closer NAC vesicle size compared with the PEG benchmark. (see Example 2)
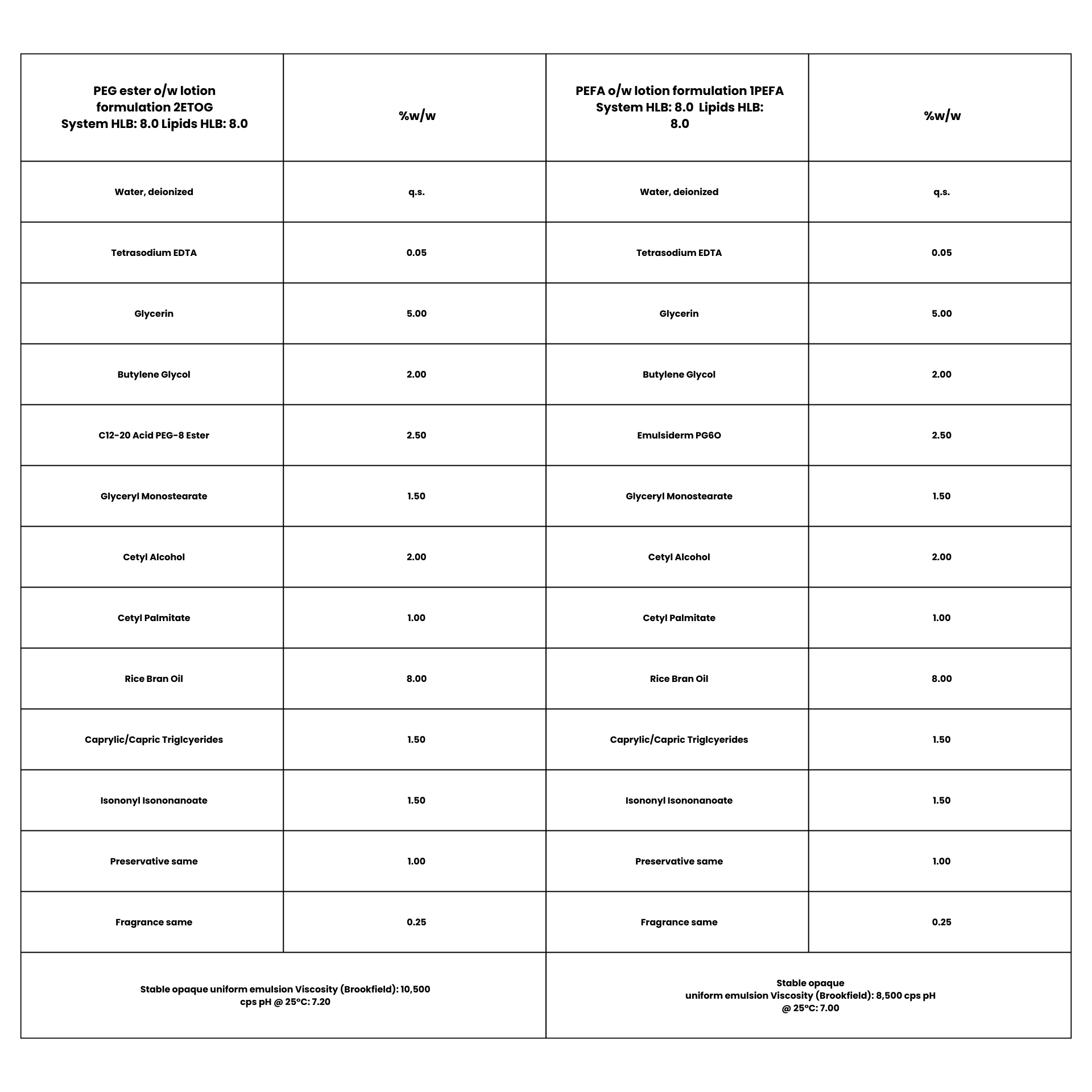

Photomicrograph of
Formulation 2ETOG
Photomicrograph of
Formulation 1PEFA
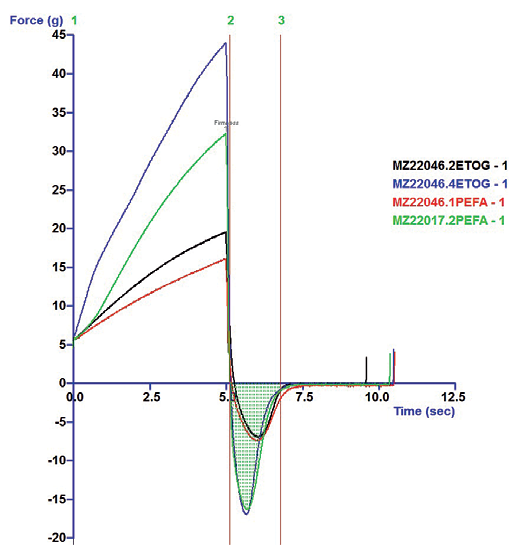
Rheometry curves for all four indicated typical pseudoplastic sheer-thinning behavior. (See Example 3) Texturometer analysis indicated the classic PEG emulsion was a firmer, more substantial lotion with greater pick-up mass, yet same uptake response, as the PEFA buddy blend. (See Example 4) These findings indicate the PEFA buddy blend formulation may have somewhat less cushioning, yet similar spreading attributes compared to the classic PEG system.
The direct surfactant-emulsifier substitution tandem with the PEGylated alkyl ester exhibited nearly complete parity with each other (see Example 4). Although the emulsion based on the primary PEFA polyglyceryl-6 oleate exhibited somewhat lower NAC on the micrograph, it shared texture and rheology attributes nearly in lockstep with the PEG ester emulsion benchmark. (see Example 4)
PEFA anomalies explained … and their prospects!
In our lengthy emulsion studies we noted some reluctance of our Emulsiderm PEFA to form lamellar liquid crystal matrices (LLC) when compared with PEGylated surfactants. We offer a theory on this dissonance and solutions to offset it. Considering the polyglyceride hydrophile end of these compounds it is apparent the high ratio of hydrogen bond donors to acceptors plays a role. This creates the condition of intramolecular hydrogen bonding between PEFA amphiphiles in solution. This interaction can lead to less affinity with water itself,11 the liquid continuum of any oil-in-water emulsion. One can say when it comes to hydrogen bonding, PEFA don’t know whether they are coming or going!
We named this phenomenon of amphiphile surfactants “bunching”, and the antidote to bunching is to increase the levels of hydrogen-bond donor polyols in the water phase. This lends a dispersive effect on the “self-absorbed” polyglycerides, levelling their distribution throughout the system and creating more numerous and uniform micelles. The greater the number and conformity of micelles in a colloidal dispersion, the more favorable that dispersion will be to LLC formation and the attendant stability achieved.
The other characteristic of PEFA contributing to more difficult LLC formation and oil loading is the polydiversity of the ester lipophile portion of the compound. PEG surfactants possess discrete lipophile locus and spatial occupancy, contributing the proximity needed for Van der Waals attraction with dispersed lipids. The more diverse juxtaposition of polyglyceride lipid ester location does not share this quality. The packing factor of correspondent PEFA will usually be less when compared to alkyl and oleyl ethoxylates.
How to manage this? We found that using the buddy system of co-emulsifiers and builders can mitigate this to a large extent. Adding a monoglyceryl or sorbitan ester will act as a “spacer” for PEFA amphiphiles, leading to higher degree of micellar packing assimilation and LLC formation. This explains why the discrete PEFA work better than the multi or mixed ester PEFA in o/w emulsion success. Employing a discrete mono-ester PEFA will create the better packing conditions with multi-ester PEFA.
Surfactant compounds based on polyglcyeryl esters of fatty acids grant the green formulator a great degree of mobility in creating new products and greener upgrades of previous formula solutions. These are based on sustainable, renewable antecedents and have exceptional safety profiles. Though seldom seen in product INCI ingredient listings before the 21st century, the term polyglyceryl is more prevalent on labels now. We encourage the chemists enabling production of green personal care and homecare products to appreciate and consider the use of these novel materials now and in the future.
References
- Structure, molecular properties: ChemSpider sponsored by Royal Society of Chemistry
- Hanno, I., Centini M., et.al. “Green Cosmetic Surfactant from Rice”, Cosmetics, 2015,2,324-341
- Babayan, V.K., et.al “Uses and Applications of Polyglycerol Esters in Cosmetic and Pharmaceutical Preparations” J. Soc. Cosmetic Chem. 15, 473-483 (1964)
- Akul Mehta, “Niosomes as Novel Drug Delivery Systems”, Pharmxchange, 26-DEC-10
- “Global Polyglyceryl Esters Market Report Forecast 2016 – 2027” QYR Research report 3270008, 08-JUL-21
- Liang Gan, et.al. “Phase Behaviour of Polyglyceryl Ester Nanoemulsions” J. Nanoscience Nanotech., 2021, 12, 6188
- USPTO #3,936,391 Gabby, et.al. to the Drackett Company, Hydrated Polyglycerol Ester Composition
- B. Neises, W. Steglich, Simple Method for Esterification of Carboxylic Acids, Agnew. Chem. Int. Ed., 1978
- Jordan, A. et.al., A Solvent Selection Guide for Steglich Esterification of Carboxylic Acids, Green Chem., 2021, 23, 6405
- Griffin, W.C., Calculation of HLB Values of Nonionic Surfactants, J. Soc. Cosmetic. Chem. 4, 249-56 (1954)
- Lockhead, R.Y. , Cosmetic Science and Technology, 2017



