
Background
Low Level alternating current (AC) or direct current (DC) microcurrent based treatment devices have been in use for over a century to improve muscle contraction, wound healing, and pain. Over the last decade the microcurrent treatment modality is increasingly being used for improvement of skin appearance, which includes skin tone, elasticity and reducing overall hyperpigmentation on face and body. To the best of our knowledge, the studies examining the benefits of non-invasive low level microcurrent treatment on cellulite have not been previously demonstrated. Cellulite is a skin condition in which the adipocyte (fat) globules aggregate and enlarge causing degradation of dermal matrix, vasoconstriction and blocking of lymphatic drainage. These enlarged hypodermal fat cells push against the dermis and epidermis resulting in a rippled or cottage cheese appearance of skin. Cellulite mostly appears in the upper triceps area of the arm, abdomen, and upper thighs. Cellulite affects women more than men. Here we report results from a 12-week clinical study using a combination of non-invasive biphasic pulsed microcurrent device system (MDS) comprising treatment body serum and post-treatment body lotion demonstrating significant improvement in appearance of cellulite on arms and upper thigh when compared to subjects who used the microcurrent device system alone.
Introduction
Cellulite is complex skin condition formed from enlarged size of fat lobules in the hypodermis and its protrusion into the papillary dermis due to the perpendicular orientation of septa, variability of thickness of septa combined with thinning and laxity of the skin resulting in alteration of the dermal architecture1. The alteration in dermal architecture is usually accompanied by loss in the density and strength of extra cellular matrix proteins and non-sulfated Glycosaminoglucans (GAGs) Hyaluronic acid. The protrusion of fat lobules in the dermis results in dimpled cottage cheese or rippled appearance. Cellulite formation predominantly occurs in women, specifically around upper tricep area of the arm, abdomen, and upper thighs. In men, the occurrence is usually noticed around the abdomen. The etiology of cellulite generally involves reduced microcirculation, interstitial lymph fluid accumulation (edema), localized enlargement of adipocytes, low grade oxidative stress and inflammation combined with changes in ECM architecture. It appears that aging increases the appearance and development of cellulite2, 3.
Cellulite formation starts occurring in women during post-adolescent phase which is exacerbated during adulthood due to increasing level of estrogen that is required to stimulate adipogenesis around the pelvis and thighs to prepare the body for pregnancy and childbirth. All women have cellulite to some degree. Obesity also exacerbates cellulite development but is not the sole cause. Non-obese and post-menopausal women also show cellulite formation. The major hormones that influence cellulite formation are estrogen and insulin4, 5. Both hormones stimulate lipogenesis in adipocytes and thus block metabolism of fat. In recent years, massage treatments with mechanical devices (Endermologie LPG)5, 6 or without devices, topicals including retinoids, methylxanthines (caffeine)7 as well as dietary supplements8 are also being used to improve cellulite.
Preferred cellulite reduction (lipolysis) approaches involve using radiofrequency and diode laser device treatments, collagenase clostridium histolyticum injections, calcium hydroxyapatite (CaHa) fillers or surgery to remove excess fat in the cellulite9. Since the treatment involves inducing lipolysis in subcutaneous fat tissue in hypodermis, topical intervention with cosmetic products alone is not effective since cosmetics can’t reach or influence the target site.
Microcurrent based non-invasive treatment devices have been gaining acceptance for improvement in skin aesthetics which includes improving skin texture, fine lines and wrinkles. Typically, the device treatment uses microcurrent output anywhere from 300 to 1000 microAmps dose which is generally safe and doesn’t scar the skin10, 11. Adverse events associated with microcurrent treatments, such as redness, usually resolve within hours. To the best of our knowledge no study showing microcurrent treatment on cellulite improvement has been demonstrated. Here we report results from a 12-week clinical study using a newly designed microcurrent device system composed of a microcurrent device delivering microcurrent output of 640 microAmps combined with a treatment gel containing lipolysis activators and a post-treatment soothing topical, henceforth referred as microcurrent device system (MDS). Using the MDS regimen 3 min, 3 times per week and 5 min, 5 times per week showed improvement in cellulite reduction and simultaneously resulted in improved skin hydration, skin tone evenness, skin firmness and elasticity. Longer duration and frequency of use resulted in more pronounced improvement in cellulite.
Objective
The purpose of this study was to examine the effects of a pulsed non-invasive microcurrent device system (MDS) in improving appearance of cellulite and skin characteristics on arms, thighs, and abdomen by comparing increased frequency and duration of use of the MDS regimen.
Methods
The study was approved by Institutional Review Boards (IRBs) and Protection of Human Subjects and followed good clinical practice guidelines. In this prospective, randomized, blinded study 60 healthy females, 25 to 60 years of age with cellulite/dimpled skin/skin laxity on thighs and arms) were randomly assigned to 2 groups (n=30/group). Group 1 used MDS (Device with a treatment topical body serum) on one assigned left or right upper thigh, upper arm, and the lower abdomen for 3 min at each location, 3 times per week; Group 2 used MDS on same locations but increased usage for 5 minutes per treatment area and 5 times per week. Both groups applied the exfoliating body cleanser in the morning each day for shower. After wiping off their body, subjects liberally applied the body serum in an assigned location on thighs, arms and abdomen followed by immediate treatment with body device with microcurrent ON for the assigned treatment time. The peanut electrode contact surface of the device was gently pressed against the body serum on the skin and glided on the body area with long upward strokes for the assigned duration. If the body serum dried up during treatment, more gel was applied. Both groups also used a post-treatment topical two times per day for all days for the 12-week study. The MDS featured an oval shaped device with four peanut shaped skin contact electrodes with opposite polarities and was optimized to deliver modulated biphasic charge balanced current output of 640 microamps. The device featured microcurrent optimization control developed using proprietary algorithm to adapt and reduce microcurrent sensations experienced by subjects at the treatment areas (Figure 1).

Skin assessments were made during visits at baseline, weeks 2, 4, 8 and 12 by expert graders, who were blinded to group assignments, using a 4-point scale (cellulite severity, depth of depression, skin surface, and laxity or skin sagginess as absent, superficial depression, medium, and deep depressions). Measurements were also made of upper thigh and upper arm circumferences (measured in the same location), firmness, and skin tone evenness. Subjects completed a self-perception questionnaire and assessments at all timepoints. Images of subject arms, abdomen and thighs were taken during each visit. In addition, skin elasticity, skin firmness, skin tone and transepidermal water loss (TEWL) were also assessed using various instruments, cutometer, chromameter and tewameter, respectively, at all timepoints. Statistical analysis of comparison between Group 1 and Group 2 were conducted using t test analysis. Statistical significance for each skin attribute was calculated from baseline to week 12 in both groups and compared between Group 1 and Group 2. Statistical significance (p<0.05) between groups (inter-group – Group 1 vs Group 2) and intra-group (baseline to week-12) was considered statistically significant.
Topical Products and Composition
- Exfoliating Body Cleanser: Formulated as a gel containing Papain, cellulose and Glycolic acid as major actives.
- Treatment Topical Body Serum: Formulated as a gel that contained Camellia Sinensis Leaf Extract, an anti-lipolytic active and Crithmum Maritimum Extract, stimulator of β-endorphins as key actives.
- Post-Treatment Topical: Developed as a lotion containing Lactic acid, Cheno-podium Quinoa Seed Extract, Hyaluronic acid and Palmitoyl Hexapeptide-12 as key actives.
Results and Discussion
Expert grader assessments showed that when compared to Group 1 there was statistically significant overall improvement (p<0.05) in cellulite appearance in Group 2 cohorts in thighs (41.7% in Group 1 vs 52.2% in Group 2) (Figure 2), Arms (41.2% in Group 1 vs 57.7% in Group 2) (Figure 3) and abdomen (32.53% in group 1 vs 65.3% in group 2) (Figure 4), at week 12 with accompanying decrease in limb circumference from baseline (p<0.05) on thighs (Figure 5), arm (Figure 6) and abdomen (Figure 7). In addition, the clinical improvement within each group at week-12 was significant compared to baseline and showed statistical improvement at 4 weeks for both groups.
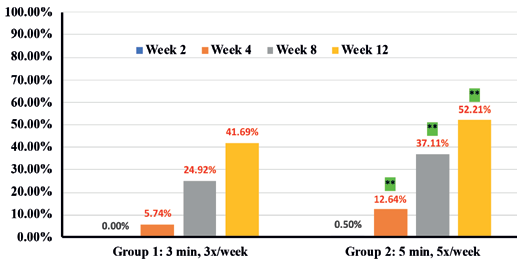
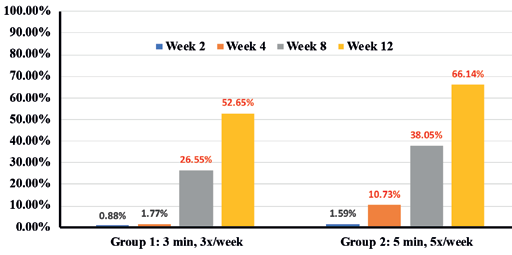
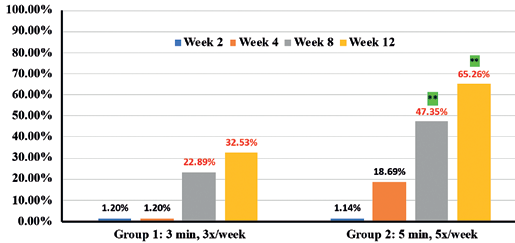
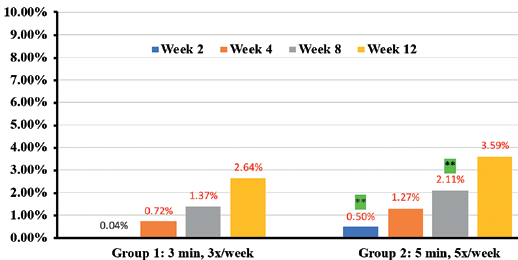
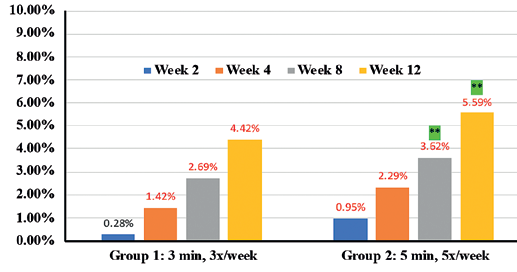
Percentages in RED indicate statistical significance over Baseline.
** indicates statistical significance Group 2 over Group 1
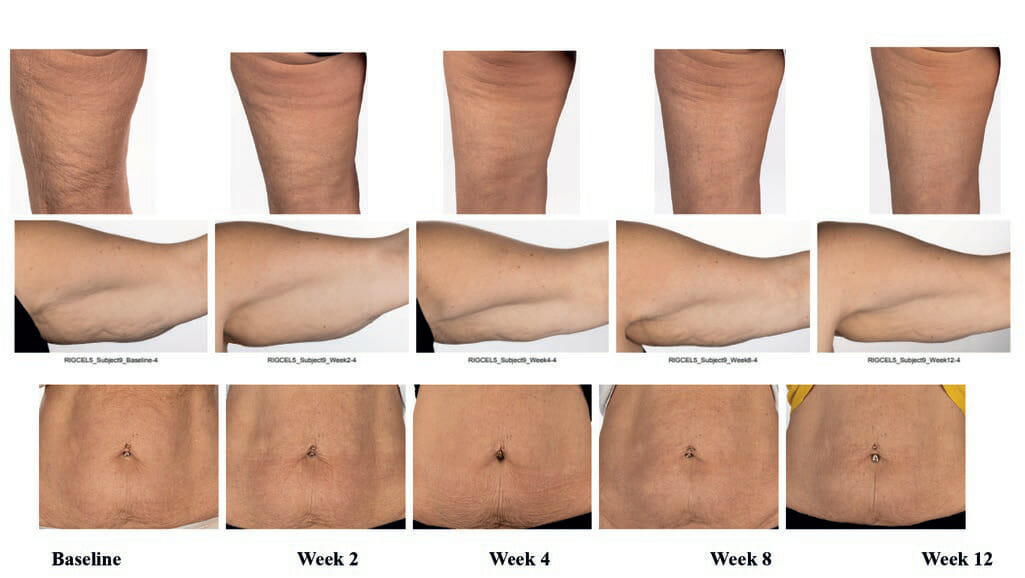
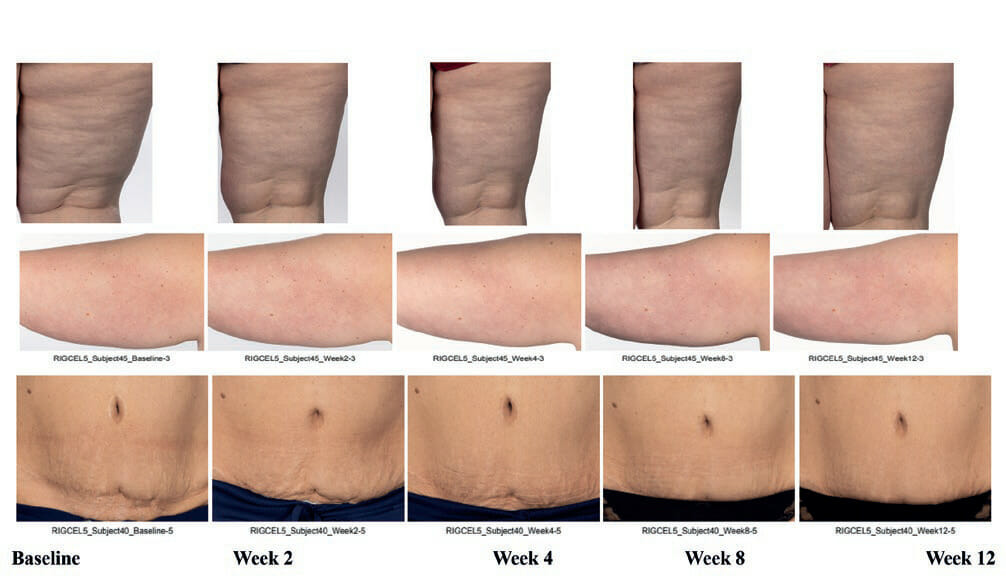
The instrumental and expert assessment correlated with imaging of subject’s thighs, arms, abdomen (not imaged in Group 1) at week 12 when compared to baseline in Group 1 (Figure 8A) and Group 2 (Figure 8B).
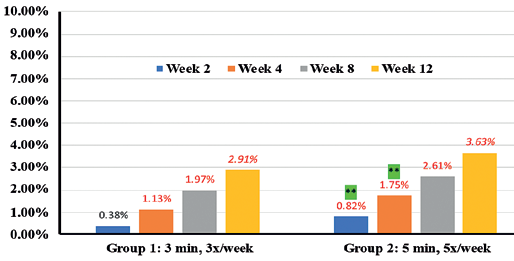
In addition, instrumental assessments showed overall improvement in trans-epidermal water loss (TEWL) (36.7% in Group 1 vs 46.6% in Group 2) (Figure 9), Skin tone evenness (4.3% in Group 1 vs 8.6% in Group 2) ) (Figure 10), Firmness (23.3% in Group 1 Resultsvs 35.9% in Group 2) (Figure 11), elasticity (23.4% in Group 1 vs 35.4% in Group 2) (Figure 12) as early as 2 weeks but greater improvement occurred over the course of the study.
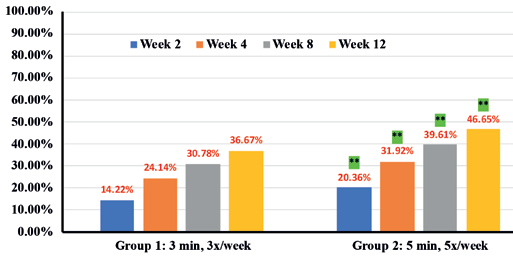
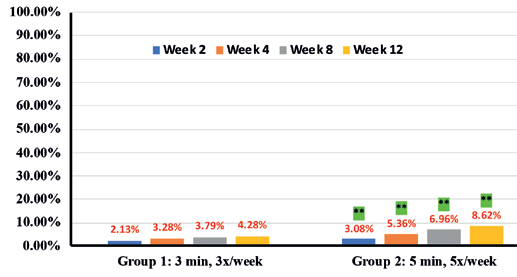
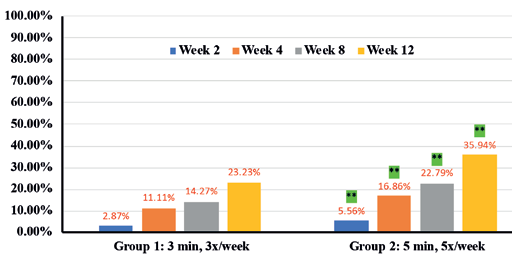
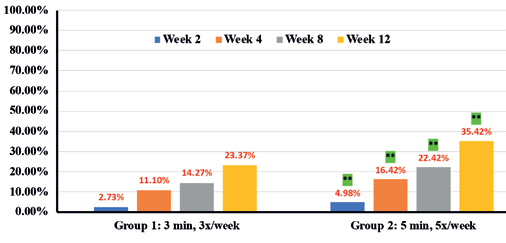
Percentages in RED indicate statistical significance over Baseline.
** indicates statistical significance Group 2 over Group 1
Self-Perception Results Comparison – Week 12 (Percentage of Subjects Improved Over Baseline)
| Skin Attributes | Group 1: 3 min, 3x/week | Group 2: 5 min, 5x/week |
|---|---|---|
| Appearance of Cellulite (Thigh) | 66.67% | 90.62% |
| Appearance of Cellulite (Arm) | 66.67% | 93.75% |
| Appearance of Cellulite (Abdomen) | 72.73% | 90.62% |
| Skin Firmness (Thigh) | 84.85% | 100.00% |
| Skin Firmness (Arm) | 84.85% | 100.00% |
| Skin Firmness (Abdomen) | 84.85% | 93.75% |
| Skin Contour (Thigh) | 72.73% | 100.00% |
| Skin Contour (Arm) | 72.73% | 100.00% |
| Skin Contour (Abdomen) | 72.73% | 100.00% |
There was a more pronounced effect on cellulite and skin attributes from week 4 to week 12 with longer and more frequent use of MDS system in Group 2 when compared to Group 1.
The instrumental and expert assessment correlated with imaging analysis of subject’s arm, abdomen, and thigh were noted at 2 weeks and became more pronounced over the course of the study when compared to baseline. Subject self-perception of cellulite improvement showed that Group 2 cohort perceived higher level improvement compared to Group 1 which included skin firmness and contouring around thigh, arm, and abdomen at week 12 (Table 1).
Conclusions
Use of MDS either for 3 minutes, 3 times per week and 5 minutes, 5 times per week on each body location – arm, thigh and abdomen improved several attributes of cellulite and skin appearance including TEWL, hydration and skin tone. Longer and more frequent use (5min, 5 times per week) of MDS produced more pronounced effects compared to 3 min, three times per week. The cellulite improvement seen in both groups suggest that in addition to reduction in fat tissue and firmer skin due to increased collagen and elastin production, improved blood circulation and increased lymphatic discharge might also be major contributing factors for cellulite reduction as observed with massaging devices such as mechanical endermologie device and with manual massage. More research is required to establish role of lymphatics in cellulite and that stimulation of lymphatic discharge being a major factor for cellulite improvement following MDS treatment.
References
1 Rossi A.B.R. and Vergnanini A.L. Cellulite: a review. J Eur Acad Dermatol & Venereol. 14, 251–262 (2000).
2 Pierard G.E, Nizet J.L. and Pierard-Franchimont C. Cellulite: from standing fat herniation to hypodermal stretch marks. Am J Dermatopathol. 22, 34–37 (2000).
3 Querleux B, Cornillon C, Jolivet O and Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 8, 118–124 (2002).
4 Goldman M.P. Cellulite: a review of current treatments. Cosmet Dermatol. 15, 17–20 (2002).
5 Kravitz L and Achenbach N.J. Cellulite: A review of its anatomy, physiology and treatment. IDEA Fitness Journal. 7(4), 36-43 (2010).
6 Chang P, Wiseman J, Jacoby T, Salisbury A.V. and Ersek R.A. Noninvasive mechanical body contouring: (Endermologie) a one-year clinical outcome study update. Aesthet Plast Surg. 2, 145–153 (1998).
7 Turati F, Pelucchi C, Marzatico F, et al. Efficacy of cosmetic products in cellulite reduction: systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 28(1), 1–15 (2014).
8 Michael S, Vivian Z, Steffen O, Ehrhardt P. Dietary Supplementation with Specific Collagen Peptides Has a Body Mass Index-Dependent Beneficial Effect on Cellulite Morphology. J Med Food. 18(12), 1340-8 (2015)
9 Neil Sadick M.D. Treatment for cellulite. International Journal of Woman’s Dermatology. 5, 68-72 (2019).
10 Kern D, Riggs M, Knaggs H.E. Biphasic modulated pulsed microcurrent treatment of skin. Tissue Regeneration and Wound Healing. 140 (7), S104 (2020).
11 Saniee F, Khademi Kalantari Kh, Yazdanpanah P, Rezasoltani A, Dabiri N, Ghafarian Shirazi HR. The effect of microcurrents on facial wrinkles. Journal of Jahrom University of Medical Sciences, 10(2), 8-15, (2012)
Acknowledgements
We thank the participants of the study and Princeton Consumer Research (PCR) United Kingdom for performing the clinical study on behalf of Nu Skin. Funding for the study was provided by Nu Skin Enterprises.
Author Contributions
Ganesh Diwakar, Helen Knaggs, Melanie Riggs, K.C. Holley conceived and developed the research idea. Melanie Riggs, K.C. Holley designed the study, supervised the data collection and analysis and in collaboration with Helen Knaggs interpreted the results.
Ganesh Diwakar, Melanie Riggs and Helen Knaggs redacted the scientific content of the manuscript, and reviewed and validated the final manuscript version.
Disclosures:
All authors are employees of Nu Skin Enterprises.



