
Introduction: The Safety Assessment in a consolidated workflow
The preparation of the PIF, CPSR and Safety Assessment 1 is a key point for the correct application of Reg. 1223/2009. Their realization, however, presents various criticalities deriving from many factors, which have been identified and focused on during a long “on field” experience of the writers. The study of the legislation in its application and of the critical issues identified, have promoted an ambitious project, which has resulted in the creation of an innovative tool, now made available to cosmetic companies by Cosmetica Italia Servizi, developed by the Angel Consulting team with the scientific supervision of the Department of Earth, Environment and Life Sciences (DISTAV) of the University of Genoa.
First of all: What is a Safety Assessment?
From the point of view of a Regulatory Area Manager, the PIFCPSR-Safety Assessment 1 is sometimes the purpose of their life. From the CEO’s perspective, it’s sometimes just a cost.
Each of them would carry out the Safety Assessment in a different way, but none of them is right, and this is why a shared and opened vision is needed.
The purpose from which the Cosmetic Product Safety Report/Safety Assessment was born is quite intuitive: the current legislation is based on self-monitoring, with a post-market survey carried out by the Competent Authorities. Among the obligations, in addition to carrying out controls and of toxicological checks, the Responsible Person must document the study path that led to the decision to place a product on the market. And here is a first answer to the starting question: the Safety Assessment is a decisionmaking tool.
A scientific, decision making, tool
But not only: in the Cosmetic Product Safety Report, the final decision (part B) is reached through the weighted, evidencebased processing of numerous scientific and toxicological data. It is therefore a scientific act, that is based on scientific data and evidence.
And here is the result of the sum of the different points of view (Regulatory, CEO, R&D), which the best organized companies have arrived at after years of development of the topic: in-depth
knowledge of the product (thanks to data on ingredients) allows us to anticipate the potential criticalities (Safe by Design) and to develop safer and more performing cosmetic products in a more rational way. Finally, the whole process of assessment procedure is documented and fulfills the legal obligations that avoid measures by the Competent Authorities.
The most current vision of the Safety Assessment is then an enhanced and preventive toxicological study. Intended (by law) to take decisions.

PIF/CPSR in the real life: challenges and matters
Obviously, the level of implementation of CPSRs in companies is also evolving, as evidenced in the course of about 150 audits carried out since 2016 by Cosmetica Italia Servizi 2, during which
we have had the opportunity to examine thousands of Product Information Files and to identify their main weaknesses.
During the Congress on Regulations and Compliance in Cosmetics (CRCC), held in 2018 3, (after our first two years of audits), the reports of some EU Competent Authorities that led the survey
on the PIFs were not far from ours: the main claimed problems were the lack of toxicological data on ingredients, on impurities (identification, expected levels and controls) and the weakness of scientific reasoning.
Furthermore, the three weaknesses are somehow interconnected, because the lack of robust toxicological data and scientific literature on ingredients hinders the identification of possible
criticalities, impurities and their expected and acceptable levels.
In the absence of this evidence, it is very difficult to structure an acceptable scientific reasoning.
A well made CPSR, the workflow, the SCCS Notes of Guidance (NoG)
The (minimum) structure of the CPSR is described in Annex I of the EU 1223/2009 regulation, but the pathway to prepare the Safety Assessment, in practice, is indicated in the SCCS Notes of
Guidance 4, which describe the well-known process: Hazard –Exposure – Dose/response – Risk calculation (with the famous and reassuring Margin of Safety, MoS, that almost all Safety Assessors dream to find ever above 100).
The same SCCS NoG also indicate the sources of information, starting from the data provided by the suppliers of the ingredients, which must be abundantly implemented with the appropriate
bibliographic literature research: “in vivo studies, in vitro and ex vivo tests, in chemico methodology, in silico methods and readacross, clinical studies, case reports, epidemiological studies and data from Post-Marketing Surveillance (PMS). […]” 4, par. 3.1
The toxicological data, implemented with the data from the literature, in fact fulfill several functions.Obviously the study of the data present in the literature allows to identify the criticality
of the substance itself, but also the production processes, the potential impurities related to them or to the degradation, and often the expected quantities of these impurities.
In addition to this, the literature data also make it possible to identify critical issues related to exposure scenarios and case histories, and last but not least, potential interference or synergies.
The process of creating the CPSR is therefore clear, which provides for an important collection of data, their comparison, the calculation of the exposure levels and the comparison between
the real exposure and the level of exposure considered safe. And which ends (we remember) with an important decision-making act. The Safety Assessment is the “green light” for the Responsible Person: it should be kept in mind.
And here the criticality becomes evident: to carry out this important and delicate process, it is essential to have a considerable amount of reliable data available, which will then have to be suitably evaluated, weighed and processed by the Safety Assessor in order to reach the conclusion, the final decision and its very important support: the scientific reasoning (also strongly based on literature data).
We must keep in mind that the Safety Assessment is a pure scientific act. But with legal implications.
The daily life of the Safety Assessor. The database.
Since the publication of Reg. 1223/2009 it has been clear that the daily life of the Safety Assessor would have involved the search, processing and management of a huge (and growing)
amount of data. Over the years, the sources of information have increased in number, and richness and complexity, as witnessed by the flow of scientific publications accessible from the most common public databases.
If on the one hand this is fundamental and decisive, on the other a further problem arises: the ever-increasing amount of work that the persons in charge must support in order to search,
discern, identify the significant endpoints, the critical points, and, therefore, process them all. For each ingredient it is easy to get lost in thousands of pages of publications, data sheets, patents,
toxicological studies, which must be rationalised, because it is unthinkable that for each product examined, the Safety Assessor could review and re-study all the copious and complex literature
he/she could find.
The need of databases was spontaneous right from the start, the structure and methods of creation and management of which soon became the subject of training courses. And it was natural for each Safety Assessor to rationalize the data found for each ingredient, in order to identify, summarize and make the most salient aspects and the most critical data usable, while (naturally) retaining the sources and documents in full version, for possible consultation more in-depth.
The solution: a common database
After the first audits of 2016, having detected the typical shortcomings in the toxicological and literature data, we easily realized that a good database, created according to well-known criteria
and well illustrated by the guidelines, created by accredited literature sources, were, in the end, very similar.
Well-prepared Safety Assessors could find more or less the same data for the same ingredients and could arrive at very similar conclusions. And from there, the idea of a common, shared,
accredited database.
Beyond the first idea: a shared, updated, endorsed, user driven, “living” and pro-active tool. Tox Tool®
The idea then underwent a further evolution. First of all, during the creation of the database, the team of Angel Consulting had the honor of participating in the creation of the Vermeer Cosmolife 5, 6, 7, in silico platform, where in the same environment were included the already exhisting and endorsed in silico tools, and further algorithm specific for the cosmetic safety assessments were developed, in order to predict the sensitization potential 6 and the NOAEL/POD4, 7.
The aims: Safety for Consumers, Safety for Companies, Safety for Safety Assessors
The Safety Assessment, it is known, due to the “green light” function for the Responsible Person, concerns a significant responsibility of the Safety Assessor, whose function (we want to clarify) the Tox Tool does not want to question in the slightest.
The supervision of a qualified expert is inviolable and constitutes, also in our opinion, the current and best way to protect consumer safety. But the amount of data and their complexity, made available by the Tox Tool, require assistance and facilitation for the very important step of decision making. Then, this is the purpose of the Tox Tool: to provide the Safety Assessor with a large amount of updated and supervised information, and to facilitate him on acquiring all the information that can support him/her in the final Assessment.
In this perspective, the safety for the Safety Assessor (and, consequently, for the company and for consumers) is increased by sharing (endorsed) data among a large group of qualified Safety
Assessors.
Endorsement
A further, no less important aspect concerns the endorsement of the database, which is carried out by a team of qualified Safety Assessors and University Professors. Considering that the realization of the Safety Assessment is a scientific act (with a legal value), the importance of a high profile scientific supervision and endorsement was considered and structured.
The Tox Tool was created with the scientific supervision of DISTAV, Department of Earth, Environment and Life Sciences of the University of Genoa, and in particular with the involvement of the team of Prof. Elena Grasselli writing here.
A further and important supervision is given by the involvement of Cosmetica Italia Servizi, a service company owned by Cosmetica Italia – Italian personal care association, which has supervised all the phases of realization and distributes it to the companies with the contribution of Dr. Marco Pirozzi, here writing.
The Reasoning
One of the major criticalities of the CPSR is undoubtedly the scientific reasoning.
It is one of the least conspicuous and most innovative elements of Reg. 1223/2009: in the previous EU directive 76/768, in fact, the Safety Assessor only had to conclude whether the product was safe or not. With Reg. 1223/2009, the Safety Assessor must add to the conclusions the scientific reasons that led him to the assessment. In order to be a real reasoning, therefore, all the critical issues must be described, many of which derive from the ingredients, projected into the exposure scenario. And the reasons why these critical issues should not produce risk must be mentioned and justified. Many of the inadequate CPSRs report, as reasoning, generic sentences such as “considering the method of manufacture … considering the impurities”. These sentences cannot be considered a “reasoning”, as it is more correct to define them as “criteria” that the Safety Assessor has applied, while a detailed motivation is requested, which in that case is missing.
From this point of view, an essential tool is offered: a summary “focus” on the most relevant critical issues related to the ingredient, which we have called “Substance Reasoning”. The tool is useful as it allows the Safety Assessor to quickly identify the major criticalities of the ingredient or the reasons why it can be considered non-dangerous, allowing for an efficient overview of the
whole. This overview constitutes the best basis for preparing the Reasoning of the finished product, which will naturally have to take into consideration all the other aspects concerning the exposure, the target and the specific scenario.
Special Feature: User Driven
The ingredients have been selected based on the frequency of use, and the most used raw materials are already present. But users can request the inclusion of missing ingredients at any time: the dedicated team will enter the data and notify the requester with a message in the account and with an email from Tox Tool team.
The Structure
The tool that was built like a dashboard, simple and intuitive to use, with all the useful information for the Safety Assessor.
The main features are:
- Shared, updated, supervised, evolving Data Base.
- User-Driven: missing data ➞ integrated at the request of users
- Additional data: confidential but always linked to the ingredients
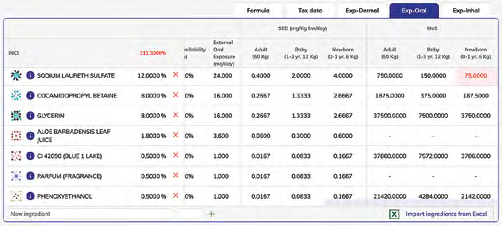
- Exposure tables ➞ MoS calculation
- Reporting and exportability: reports in .pdf or raw excel data
- Protection: proprietary server, reporting with QR-code that
associates the reports with the owner company - Reasoning: per each ingredient, a reasoning is given
The landing page: from the website www.toxtool.eu:

Ingredients – browsing at three levels of sight:
Overview (with scrolling subchapters) and popups for “flash” view on the specific detail.
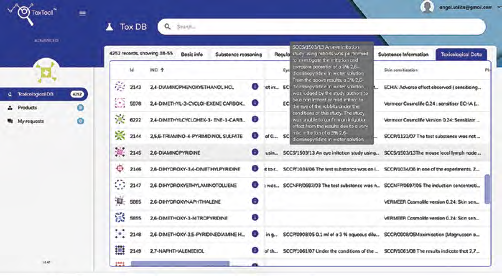
Overview on the specific ingredient:
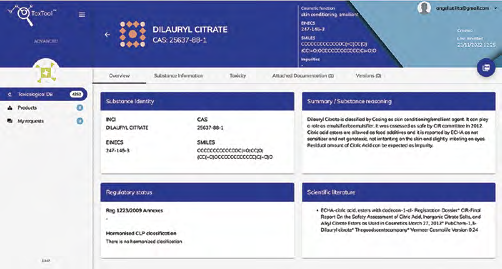
Monography (pdf)


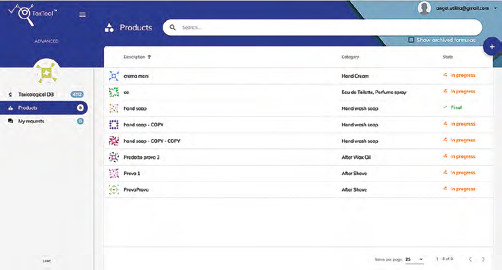
Final Products (reserved area)
Formula imported from excel:
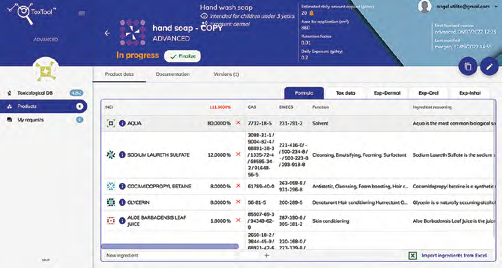
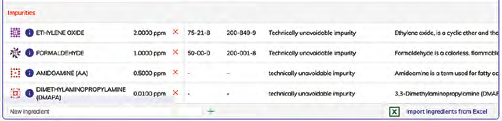
Expected Impurities are considered and included in calculations.
Exposure with MoS calculation:
Reporting:
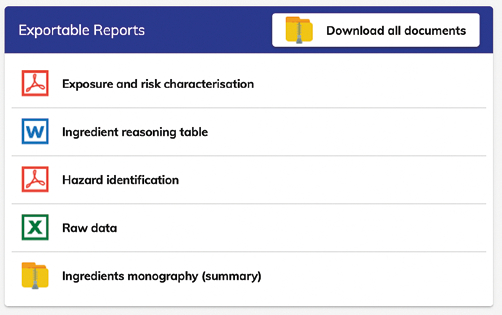
The reports that can be easily downloaded are in .pdf except the Raw data (an Excel file is given) and Ingredient reasoning table that is in word, as it can be easily copied for being part of the final product Reasoning.
The Tox Tool®, today, and the future
After months of beta-testing, Tox Tool was presented to companies on October 13th, and is currently functional and used by a constantly growing number of companies. In addition to the functionality, obviously much appreciated, to carry out CPSR and Safety Assessment, it turns out to be a valid tool for quick and preliminary stage verification in advanced R&D projects.
Due to the way it was conceived, and due to the large team of experts constantly involved, the tool will be subject to constant evolution, both for the number of ingredients included, and for
the addition of new endpoints and new data sources.
In particular, implementations are planned to support the new Next Generation Risk Assessment approach (NGRA) presented in the XI edition of the SCCS Notes of Guidance [4].
References
- Definitions, under EU 1223/2009 Regulation, PIF: Product Information File (Art. 11), CPSR: Cosmetic Product Safety Report (Annex I), Safety Assessment: Cosmetic Product Safety Assessment (Art.10).
- Cosmetica Italia Servizi, owned by Italian cosmetic companies association, Cosmetica Italia, provides services and among them audits to the companies, in order to check the compliance and to individuate weaknesses, called “check-up”.
- The 3rd International Congress on Regulations and Compliance in Cosmetics (CRCC) 22 – 23 October 2018 Brussels, Belgium
- SCCS/1628/21: SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation – 11th revision https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic- ingredients-and-their-safety-evaluation-11th-revision_en
- LIFE-Vermeer, Integrating VEGA, ToxRead, MERLIN-Expo, and ERICA in a platform for risk assessment and substitution of risky substance. LIFE16 ENV/IT/000167 https://www.life-vermeer.eu/download-software/software- vermeer-cosmolife/
- Selvestrel G, Robino F, Russo MZ. In Silico Models for Skin Sensitization and Irritation. Methods Mol Biol. 2022;2425:291-354. doi: 10.1007/978-1-0716- 1960-5_13. PMID: 35188638.
- Selvestrel G, Robino F, Baderna D, Manganelli S, Asturiol D, Manganaro A, Zanotti Russo M, Lavado G, Toma C, Roncaglioni A, Benfenati E. Sphera-Cosmolife: a new tool for the risk assessment of cosmetic products. ALTEX. 2021;38(4):565-579. doi: 10.14573/altex.2010221. Epub 2021 May 3. PMID: 33963416.



